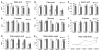Comparative transcriptomics and proteomics analysis of citrus fruit, to improve understanding of the effect of low temperature on maintaining fruit quality during lengthy post-harvest storage - PubMed (original) (raw)
Comparative Study
. 2012 May;63(8):2873-93.
doi: 10.1093/jxb/err390. Epub 2012 Feb 8.
Affiliations
- PMID: 22323274
- PMCID: PMC3350911
- DOI: 10.1093/jxb/err390
Comparative Study
Comparative transcriptomics and proteomics analysis of citrus fruit, to improve understanding of the effect of low temperature on maintaining fruit quality during lengthy post-harvest storage
Ze Yun et al. J Exp Bot. 2012 May.
Abstract
Fruit quality is a very complex trait that is affected by both genetic and non-genetic factors. Generally, low temperature (LT) is used to delay fruit senescence and maintain fruit quality during post-harvest storage but the molecular mechanisms involved are poorly understood. Hirado Buntan Pummelo (HBP; Citrus grandis × C. paradis) fruit were chosen to explore the mechanisms that maintain citrus fruit quality during lengthy LT storage using transcriptome and proteome studies based on digital gene expression (DGE) profiling and two-dimensional gel electrophoresis (2-DE), respectively. Results showed that LT up-regulated stress-responsive genes, arrested signal transduction, and inhibited primary metabolism, secondary metabolism and the transportation of metabolites. Calcineurin B-like protein (CBL)-CBL-interacting protein kinase complexes might be involved in the signal transduction of LT stress, and fruit quality is likely to be regulated by sugar-mediated auxin and abscisic acid (ABA) signalling. Furthermore, ABA was specific to the regulation of citrus fruit senescence and was not involved in the LT stress response. In addition, the accumulation of limonin, nomilin, methanol, and aldehyde, together with the up-regulated heat shock proteins, COR15, and cold response-related genes, provided a comprehensive proteomics and transcriptomics view on the coordination of fruit LT stress responses.
Figures
Fig. 1.
Distribution of identified proteins on two-dimensional gel. Differential accumulation analysis was carried out using PDQuest software. Those spots with more than two-fold difference in accumulation over three independently analysed gels were removed from identified gels for MS/MS analysis. The master gel was used for the distribution of successful identified proteins. Red arrows indicate that proteins were up-regulated by low-temperature storage stress compared to ambient-temperature storage. Green arrows indicate that proteins were down-regulated by low-temperature storage stress. Blue arrows indicate that the proteins changed during storage. (This figure is available in colour at JXB online).
Fig. 2.
Representative two-dimensional electrophoresis profiles of proteins from Hirado Buntan Pummelo during post-harvest under ambient temperature and low temperature. The juice sacs from two segments of ten individual fruits were sampled at 24, 48, 72, 96, and 120 days after harvest (DAH), A total of ten samples were harvested from the two storage conditions at 24 d intervals from 3 December 2008 to 1 April 2009. Proteins were extracted using phenol extraction protocol. Proteins (100 μg) were separated in the first dimension on IPG strip (17 cm, pH 4–7) and in the second dimension on a 15% SDS-PAGE gel. Gels were visualized by silver staining.
Fig. 3.
Functional categorization of the low-temperature-storage-specific accumulated proteins in Hirado Buntan Pummelo fruit juice sacs. MS data was searched against the Viridiplantae non-redundant protein database at the NCBI homepage (
http://www.ncbi.nlm.nih.gov/blast/Blast.cgi
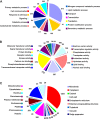
) using MASCOT software. A total of 63 proteins were annotated using gene ontology and categorized according to protein function (A), molecular function (B), and cellular component (C). (This figure is available in colour at JXB online).
Fig. 4.
Trends in organic acid and soluble sugar contents in Hirado Buntan Pummelo juice sacs (fresh weight) during post-harvest storage. Malic acid, citric acid, quinic acid, fructose, glucose, and sucrose contents were extracted using 80% methanol and measured using gas chromatography at 24, 48, 72, 96, and 120 d after harvest (DAH). Three individual replicates were used to reduce experimental error. Total organic acid (TOA) and total soluble sugar (TSS) were the sum of the organic acids (malic acid, citric acid, and quinic acid) and the soluble sugars (fructose, glucose, and sucrose), respectively. The accumulation under AT is shown by a white column and the accumulation under LT is shown by a gray column. Different letters (A, B, C, and D) represent statistically significant differences (P < 0.01), analysed using Student’s t-test.
Fig. 5.
Indexes of metabolites of secondary metabolism and anaerobic respiration during ambient temperature (AT) and low temperature (LT) storage. Naringin concentration was determined by a method that used solid-phase sample preparation and HPLC. Nomilin and limonin were extracted with 15 ml CH2Cl2 in a Soxhlet extractor and measured using HPLC. Aldehyde, alcohol, and methanol were volatilized using a 65 °C water bath and detected using GC. (A–C) Specific accumulations of naringin, nomilin, and limonin in Hirado Buntan Pummelo juice sacs at 24, 48, 72, 96, and 120 d after harvest (DAH). (D–F) Specific accumulations of aldehyde, alcohol, and methanol. Fresh-weight samples were used. The accumulation under AT is shown by a white column and the accumulation under LT is shown by a gray column. Different letters (A, B, C, and D) represent statistically significant differences (P < 0.01), analysed using Student’s t-test. (This figure is available in colour at JXB online.)
Fig. 6.
Functional categorization of the genes with significant transcriptional changes between ambient temperature (AT) and low temperature (LT) conditions. Total RNA was extracted from Hirado Buntan Pummelo fruit at 72 DAH under AT and LT stress. After the comparative analysis of digital gene expression profiling, differential expressed genes were annotated using gene ontology and categorized according to biological process (A), molecular function (B), and cellular component (C).
Fig. 7.
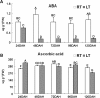
Abscisic acid (ABA) (A) and ascorbic acid (B) contents under ambient temperature (AT) and low temperature (LT). ABA and ascorbic acid was extracted from Hirado Buntan Pummelo juice sacs (fresh fruit) 24, 48, 72, 96, and 120 days after harvest (DAH). Both were determined using HPLC analysis with a C18 column. The absorbances of ABA and ascorbic acid were 262 nm and 243 nm, respectively. Three individual replicates were used to reduce experimental error. The accumulation at AT is shown by a white column and the accumulation under LT is shown by a gray column. Different letters (A, B, C, and D) represent statistically significant differences (P < 0.01), analysed using Student’s t-test.
Fig. 8.
Transcript levels of 28 selected genes at different stages of fruit storage under AT and LT. The relative expression levels were analysed by quantitative real-time reverse-transcription PCR. The gene expression under AT is shown by a white column and the gene expression under LT is shown by a gray column. Different letters (A, B, C, and D) represent statistically significant differences (P < 0.01), analysed using Student’s t-test.
Similar articles
- Comprehensive insights on how 2,4-dichlorophenoxyacetic acid retards senescence in post-harvest citrus fruits using transcriptomic and proteomic approaches.
Ma Q, Ding Y, Chang J, Sun X, Zhang L, Wei Q, Cheng Y, Chen L, Xu J, Deng X. Ma Q, et al. J Exp Bot. 2014 Jan;65(1):61-74. doi: 10.1093/jxb/ert344. Epub 2013 Nov 9. J Exp Bot. 2014. PMID: 24215076 Free PMC article. - Integration of metabolomics and subcellular organelle expression microarray to increase understanding the organic acid changes in post-harvest citrus fruit.
Sun X, Zhu A, Liu S, Sheng L, Ma Q, Zhang L, Nishawy EM, Zeng Y, Xu J, Ma Z, Cheng Y, Deng X. Sun X, et al. J Integr Plant Biol. 2013 Nov;55(11):1038-53. doi: 10.1111/jipb.12083. J Integr Plant Biol. 2013. PMID: 23758915 - Comparative proteomic and metabolomic profiling of citrus fruit with enhancement of disease resistance by postharvest heat treatment.
Yun Z, Gao H, Liu P, Liu S, Luo T, Jin S, Xu Q, Xu J, Cheng Y, Deng X. Yun Z, et al. BMC Plant Biol. 2013 Mar 16;13:44. doi: 10.1186/1471-2229-13-44. BMC Plant Biol. 2013. PMID: 23497220 Free PMC article. - Transcriptome profiling of postharvest strawberry fruit in response to exogenous auxin and abscisic acid.
Chen J, Mao L, Lu W, Ying T, Luo Z. Chen J, et al. Planta. 2016 Jan;243(1):183-97. doi: 10.1007/s00425-015-2402-5. Epub 2015 Sep 15. Planta. 2016. PMID: 26373937 - Advances of section drying in citrus fruit: The metabolic changes, mechanisms and prevention methods.
Kang C, Cao J, Wang Y, Sun C. Kang C, et al. Food Chem. 2022 Nov 30;395:133499. doi: 10.1016/j.foodchem.2022.133499. Epub 2022 Jun 16. Food Chem. 2022. PMID: 35802975 Review.
Cited by
- Effects of Near-Freezing Temperature Combined with Jujube Polysaccharides Treatment on Proteomic Analysis of 'Diaogan' Apricot (Prunus armeniaca L.).
Wang Z, Wang W, Li W, Yang R, Li Y, Zhang L, Zhang M, Li X. Wang Z, et al. Foods. 2023 Dec 16;12(24):4504. doi: 10.3390/foods12244504. Foods. 2023. PMID: 38137308 Free PMC article. - Study on peanut protein oxidation and metabolomics/proteomics analysis of peanut response under hypoxic/re-aeration storage.
Li W, Zhou Y, Zhang H, Hu M, Lu P, Qu C. Li W, et al. Food Chem X. 2024 Feb 6;21:101173. doi: 10.1016/j.fochx.2024.101173. eCollection 2024 Mar 30. Food Chem X. 2024. PMID: 38370304 Free PMC article. - Cold and Heat Stress Diversely Alter Both Cauliflower Respiration and Distinct Mitochondrial Proteins Including OXPHOS Components and Matrix Enzymes.
Rurek M, Czołpińska M, Pawłowski TA, Krzesiński W, Spiżewski T. Rurek M, et al. Int J Mol Sci. 2018 Mar 16;19(3):877. doi: 10.3390/ijms19030877. Int J Mol Sci. 2018. PMID: 29547512 Free PMC article. - Transcriptome analysis of ripe and unripe fruit tissue of banana identifies major metabolic networks involved in fruit ripening process.
Asif MH, Lakhwani D, Pathak S, Gupta P, Bag SK, Nath P, Trivedi PK. Asif MH, et al. BMC Plant Biol. 2014 Dec 2;14:316. doi: 10.1186/s12870-014-0316-1. BMC Plant Biol. 2014. PMID: 25442405 Free PMC article. - Effect of temperature treatment on fruit quality and immunoregulation of Satsuma (Citrus unshiu Marc.) during storage.
Deng W, Wu J, Da Y, Ma Z. Deng W, et al. Food Sci Nutr. 2020 Aug 30;8(10):5443-5451. doi: 10.1002/fsn3.1771. eCollection 2020 Oct. Food Sci Nutr. 2020. PMID: 33133547 Free PMC article.
References
- Alexander L, Grierson D. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. Journal of Experimental Botany. 2002;53:2039–2055. - PubMed
- Allen G, Chu S, Harrington C, Schumacher K, Hoffmann T, Tang Y, Grill E, Schroeder J. A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature. 2001;411:1053–1057. - PubMed
- Alos E, Roca M, Iglesias DJ, Minguez-Mosquera MI, Damasceno CMB, Thannhauser TW, Rose JKC, Talon M, Cercos M. An evaluation of the basis and consequences of a stay-green mutation in the navel negra citrus mutant using transcriptomic and proteomic profiling and metabolite analysis. Plant Physiology. 2008;147:1300–1315. - PMC - PubMed
- Ashraf M, Akram NA, Arteca RN, Foolad MR. The physiological, biochemical and molecular roles of brassinosteroids and salicylic acid in plant processes and salt tolerance. Critical Reviews in Plant Sciences. 2010;29:162–190.
- Audic S, Claverie JM. The significance of digital gene expression profiles. Genome Research. 1997;7:986–995. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous