Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland - PubMed (original) (raw)
Review
Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland
Shannon L Kelleher et al. Adv Nutr. 2011 Mar.
Abstract
Zinc (Zn) is an essential micronutrient required for over 300 different cellular processes, including DNA and protein synthesis, enzyme activity, and intracellular signaling. Cellular Zn homeostasis necessitates the compartmentalization of Zn into intracellular organelles, which is tightly regulated through the integration of Zn transporting mechanisms. The pancreas, prostate, and mammary gland are secretory tissues that have unusual Zn requirements and thus must tightly regulate Zn metabolism through integrating Zn import, sequestration, and export mechanisms. Recent findings indicate that these tissues utilize Zn for basic cellular processes but also require Zn for unique cellular needs. In addition, abundant Zn is transported into the secretory pathway and a large amount is subsequently secreted in a tightly regulated manner for unique biological processes. Expression of numerous members of the SLC30A (ZnT) and SLC39A (Zip) gene families has been documented in these tissues, yet there is limited understanding of their precise functional role in Zn metabolism or their regulation. Impairments in Zn secretion from the pancreas, prostate, and mammary gland are associated with disorders such as diabetes, infertility, and cancer, respectively. In this review, we will provide a brief summary of the specific role of Zn in each tissue and describe our current knowledge regarding how Zn metabolism is regulated. Finally, in each instance, we will reflect upon how this information shapes our current understanding of the role of Zn in these secretory tissues with respect to human health and disease.
Figures
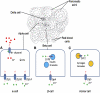
Figure 1
Zn transport in various pancreatic cell types. (A) Localization of Zip1, Zip10, and Zip14 to pancreatic α-cells suggests that these transporters are responsible for importing Zn into the cell. Zn binds to and opens ATP dependent K(+) channels, allowing the efflux of Zn from the α-cell and inactivation of voltage dependent calcium channels, resulting in decreased glucagon secretion. (B) Zn is transported into pancreatic β-cell cells via Zip4. ZnT8 is responsible for the transport of Zn into insulin granules. Autoantibodies to ZnT8 and polymorphisms of ZnT8 are associated with the onset of DM. (C) Zip5 is responsible for the transport of Zn into pancreatic acinar cells. Zn is transported into zymogen granules by ZnT2 where it binds to and activates digestive enzymes that are subsequently secreted.
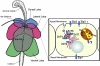
Figure 2
Zn transporter expression in the secreting epithelium of the rat prostate. Lobes of the rat prostate are illustrated on the left. On the right, a cartoon of an epithelial cell of the lateral lobe illustrates our current understanding of Zn transporter localization and Zn function. Zip2 and Zip3 are localized to the apical membrane and Zip1 is localized to the basal membrane; all function to import Zn into the cell. ZnT4, ZnT6, and ZnT7 are localized to the Golgi apparatus and ZnT2 is localized to the ER; these transporters likely function to maintain the secretory pathway. There is no known transporter for the mitochondria where high Zn levels inhibit the oxidation of citrate, providing a source for secretion.
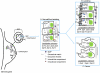
Figure 3
Zn and Zn transporters involved in mammary gland function. Mammary gland is comprised of a network of branched ductal structures terminating at acinar units, also known as lobules. These structures are lined with MEC responsible for the production and secretion of milk into the collecting ducts for the developing neonate. (A) Although only localization of Zip3 and ZnT2 has been clearly established in normal/nonlactating tissue, ZnT4 is also known to be expressed. Zip6 and Zip7 are expressed in breast cells; however, their role in normal mammary gland is unknown. (B) During lactation, ZnT2 plays a major role in the secretion of Zn into milk, while Zip3 plays a role in the reuptake of Zn from the lumen. The localization and contribution of other Zn transporters during MEC differentiation and lactation remains to be determined and is an important question. (C) Hyper-accumulation of Zn has been associated with breast cancer. A limited number of Zn transporters (Zip5, Zip6, Zip7, Zip8, and Zip10) and MT have been shown to be overexpressed in this disease. However, their specific contribution to this phenotype and in MEC is remains to be delineated.
Similar articles
- Mapping the zinc-transporting system in mammary cells: molecular analysis reveals a phenotype-dependent zinc-transporting network during lactation.
Kelleher SL, Velasquez V, Croxford TP, McCormick NH, Lopez V, MacDavid J. Kelleher SL, et al. J Cell Physiol. 2012 Apr;227(4):1761-70. doi: 10.1002/jcp.22900. J Cell Physiol. 2012. PMID: 21702047 Free PMC article. - Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis.
Liuzzi JP, Bobo JA, Lichten LA, Samuelson DA, Cousins RJ. Liuzzi JP, et al. Proc Natl Acad Sci U S A. 2004 Oct 5;101(40):14355-60. doi: 10.1073/pnas.0406216101. Epub 2004 Sep 20. Proc Natl Acad Sci U S A. 2004. PMID: 15381762 Free PMC article. - The biology of zinc transport in mammary epithelial cells: implications for mammary gland development, lactation, and involution.
McCormick NH, Hennigar SR, Kiselyov K, Kelleher SL. McCormick NH, et al. J Mammary Gland Biol Neoplasia. 2014 Mar;19(1):59-71. doi: 10.1007/s10911-013-9314-4. Epub 2013 Dec 15. J Mammary Gland Biol Neoplasia. 2014. PMID: 24338187 Review. - Zip3 plays a major role in zinc uptake into mammary epithelial cells and is regulated by prolactin.
Kelleher SL, Lönnerdal B. Kelleher SL, et al. Am J Physiol Cell Physiol. 2005 May;288(5):C1042-7. doi: 10.1152/ajpcell.00471.2004. Epub 2005 Jan 5. Am J Physiol Cell Physiol. 2005. PMID: 15634741 - Molecular regulation of milk trace mineral homeostasis.
Kelleher SL, Lönnerdal B. Kelleher SL, et al. Mol Aspects Med. 2005 Aug-Oct;26(4-5):328-39. doi: 10.1016/j.mam.2005.07.005. Mol Aspects Med. 2005. PMID: 16098572 Review.
Cited by
- Patterns and Associations of Essential Trace Elements (Cu, Fe and Zn) in Saudi Adults with Varying Levels of Glycemia.
Yakout S, Faqeeh F, Al-Attas O, Hussain SD, Al-Daghri NM. Yakout S, et al. Metabolites. 2021 May 6;11(5):297. doi: 10.3390/metabo11050297. Metabolites. 2021. PMID: 34066324 Free PMC article. - Cellular mechanisms of zinc dysregulation: a perspective on zinc homeostasis as an etiological factor in the development and progression of breast cancer.
Alam S, Kelleher SL. Alam S, et al. Nutrients. 2012 Aug;4(8):875-903. doi: 10.3390/nu4080875. Epub 2012 Jul 30. Nutrients. 2012. PMID: 23016122 Free PMC article. Review. - An analysis of benign human prostate offers insights into the mechanism of apocrine secretion and the origin of prostasomes.
Fullwood NJ, Lawlor AJ, Martin-Hirsch PL, Matanhelia SS, Martin FL. Fullwood NJ, et al. Sci Rep. 2019 Mar 14;9(1):4582. doi: 10.1038/s41598-019-40820-2. Sci Rep. 2019. PMID: 30872668 Free PMC article. - The zinc transporter Zip5 (Slc39a5) regulates intestinal zinc excretion and protects the pancreas against zinc toxicity.
Geiser J, De Lisle RC, Andrews GK. Geiser J, et al. PLoS One. 2013 Nov 26;8(11):e82149. doi: 10.1371/journal.pone.0082149. eCollection 2013. PLoS One. 2013. PMID: 24303081 Free PMC article. - PUMA: a unified framework for penalized multiple regression analysis of GWAS data.
Hoffman GE, Logsdon BA, Mezey JG. Hoffman GE, et al. PLoS Comput Biol. 2013;9(6):e1003101. doi: 10.1371/journal.pcbi.1003101. Epub 2013 Jun 27. PLoS Comput Biol. 2013. PMID: 23825936 Free PMC article.
References
- Eide DJ. Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta. 2006;1763:711–22 - PubMed
- Palmiter RD, Huang L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. : Hediger MA, editor The ABC of solute carriers. New York: Springer-Verlag; 2003 - PubMed
- Kambe T, Narita H, Yamaguchi-Iwai Y, Hirose J, Amano T, Sugiura N, Sasaki R, Mori K, Iwanaga T, et al. Cloning and characterization of a novel mammalian zinc transporter, zinc transporter 5, abundantly expressed in pancreatic beta cells. J Biol Chem. 2002;277:19049–55 - PubMed
- Dufner-Beattie J, Langmade SJ, Wang F, Eide D, Andrews GK. Structure, function, and regulation of a subfamily of mouse zinc transporter genes. J Biol Chem. 2003;278:50142–50 - PubMed
- Huber AM, Gershoff SN. Effect of zinc deficiency in rats on insulin release from the pancreas. J Nutr. 1973;103:1739–44 - PubMed