SEL1L, an UPR response protein, a potential marker of colonic cell transformation - PubMed (original) (raw)
doi: 10.1007/s10620-011-2026-y. Epub 2012 Feb 16.
William Green, Giovanna Finzi, Fausto Sessa, Mehdi Nouraie, Edward L Lee, Annalisa Morgano, Antonio Moschetta, Monica Cattaneo, Renato Mariani-Costantini, Hassan Brim, Ida Biunno
Affiliations
- PMID: 22350780
- PMCID: PMC3345950
- DOI: 10.1007/s10620-011-2026-y
SEL1L, an UPR response protein, a potential marker of colonic cell transformation
Hassan Ashktorab et al. Dig Dis Sci. 2012 Apr.
Abstract
Background: SEL1L gene product is implicated in the endoplasmic reticulum (ER)-associated protein degradation and Unfolded Protein Response pathways. This gene and associated miRNAs have been indicated as predictive and prognostic markers of pancreatic cancer.
Aim: Explore the role of SEL1L in colorectal cancer (CRC) progression.
Methods: SEL1L expression was analysed immunohistochemically in 153 adenomas and 71 CRCs from African American and North Italian patients. The distribution of stained cells was determined by computing median and inter quartile range. The receiver operating characteristics plot was used as discriminate power of SEL1L expression, CRC diagnosis and the effects on patient survival.
Results: SEL1L was low in normal mucosa and confined to few scattered cells at the base crypt of the villi and in the foveolar glandular compartment. The highest levels were in Paneth cells within the lysosomes. The enterocytic progenitor cells and mature enterocytes showed less cytoplasmic staining. In CRCs, SEL1L expression significantly correlated with the progression from adenoma to carcinoma (P = 0.0001) being stronger in well-to-moderately differentiated cancers. No correlation was found with other clinicopathological characteristics or ethnicity.
Conclusions: SEL1L expression is a potential CRC tissue biomarker since its expression is significantly higher in adenoma cells with respect to normal mucosa. The levels of expression decrease sensibly in undifferentiated CRC cancers. Interestingly, Paneth cells contain high levels of SEL1L protein that could indicate pre-neoplastic mucosa undergoing neoplastic transformation. Since SEL1L's major function lies within ER stress and active ERAD response, it may identify CRCs with differentiated secretory phenotype and acute cellular stress.
Conflict of interest statement
Conflict of Interest None
Figures
Fig. 1
Western blot analysis for SEL1LA of the p38 SEL1L protein lysates obtained from normal tissue mucosa, adenoma and adenocarcinoma from four CRC patients. a An increase of SEL1LA, the ER-resident form of SEL1L, in the adenoma (A) and adenocarcinoma (T) cells when compared to normal mucosa (M) was noted in all four patients (a, lanes: patient 1-A, patient 2-T, patient 3-M and patient 4-A). In addition, a remarkable increase of the p38 SEL1L variant was seen in the neoplastic protein extracts. b Fold of SEL1L proteins increase in the neoplastic versus the normal mucosa cells, the levels were normalized toward the housekeeping p85 protein. Occasionally the p38 variant of SEL1L showed the presence of a post-translationally modified form
Fig. 2
a–j SEL1L expression in CRC progression. a, b Left, Normal colon with decreasing staining ascending the crypt 20X. Right, Normal colon base of crypt (proliferative zone) with cytoplasmic staining, 40X. c, d Left, Cytoplasmic staining in adenoma, 40X. Right, Nuclear staining in adenoma, 40X. e, f Left, Well-differentiated carcinoma, 10X. Right, 40X. g, h Moderately differentiated carcinoma, 10X. Right, 40X. i, j Left, Poorly differentiated carcinoma 10X. Right, 40X
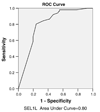
Fig. 3
ROC curve for SEL1L as biomarker for colorectal cancer diagnosis
Fig. 4
SEL1L immunoreactivity in intestinal mucosa Paneth’s cells both at optical (right bottom inset) and ultrastructural level. The electron microscopy shows that the immunoreactivity is concentrated in the secretory granules, as best shown in right top inset by the immunogold technique
Similar articles
- REG IV overexpression in an early stage of colorectal carcinogenesis: an immunohistochemical study.
Li XH, Zheng Y, Zheng HC, Takahashi H, Yang XH, Masuda S, Takano Y. Li XH, et al. Histol Histopathol. 2010 Apr;25(4):473-84. doi: 10.14670/HH-25.473. Histol Histopathol. 2010. PMID: 20183800 - Expression of ATF6 as a marker of pre-cancerous atypical change in ulcerative colitis-associated colorectal cancer: a potential role in the management of dysplasia.
Hanaoka M, Ishikawa T, Ishiguro M, Tokura M, Yamauchi S, Kikuchi A, Uetake H, Yasuno M, Kawano T. Hanaoka M, et al. J Gastroenterol. 2018 May;53(5):631-641. doi: 10.1007/s00535-017-1387-1. Epub 2017 Sep 7. J Gastroenterol. 2018. PMID: 28884228 Free PMC article. - The clinicopathological significance of REIC expression in colorectal carcinomas.
Wang W, Zhu W, Xu XY, Nie XC, Yang X, Xing YN, Yu M, Liu YP, Takano Y, Zheng HC. Wang W, et al. Histol Histopathol. 2012 Jun;27(6):735-43. doi: 10.14670/HH-27.735. Histol Histopathol. 2012. PMID: 22473694 - Logarithmic expansion of LGR5+ cells in human colorectal cancer.
Martin ML, Zeng Z, Adileh M, Jacobo A, Li C, Vakiani E, Hua G, Zhang L, Haimovitz-Friedman A, Fuks Z, Kolesnick R, Paty PB. Martin ML, et al. Cell Signal. 2018 Jan;42:97-105. doi: 10.1016/j.cellsig.2017.09.018. Epub 2017 Sep 25. Cell Signal. 2018. PMID: 28958617 Free PMC article. - Colorectal carcinogenesis: a cellular response to sustained risk environment.
Fung KY, Ooi CC, Zucker MH, Lockett T, Williams DB, Cosgrove LJ, Topping DL. Fung KY, et al. Int J Mol Sci. 2013 Jun 27;14(7):13525-41. doi: 10.3390/ijms140713525. Int J Mol Sci. 2013. PMID: 23807509 Free PMC article. Review.
Cited by
- Protein Quality Control in the Endoplasmic Reticulum and Cancer.
Moon HW, Han HG, Jeon YJ. Moon HW, et al. Int J Mol Sci. 2018 Oct 3;19(10):3020. doi: 10.3390/ijms19103020. Int J Mol Sci. 2018. PMID: 30282948 Free PMC article. Review. - Microbiome analysis of stool samples from African Americans with colon polyps.
Brim H, Yooseph S, Zoetendal EG, Lee E, Torralbo M, Laiyemo AO, Shokrani B, Nelson K, Ashktorab H. Brim H, et al. PLoS One. 2013 Dec 20;8(12):e81352. doi: 10.1371/journal.pone.0081352. eCollection 2013. PLoS One. 2013. PMID: 24376500 Free PMC article. - Unfolded Protein Response of the Endoplasmic Reticulum in Tumor Progression and Immunogenicity.
Yoo YS, Han HG, Jeon YJ. Yoo YS, et al. Oxid Med Cell Longev. 2017;2017:2969271. doi: 10.1155/2017/2969271. Epub 2017 Dec 21. Oxid Med Cell Longev. 2017. PMID: 29430279 Free PMC article. Review. - Cell and tissue microarray technologies for protein and nucleic acid expression profiling.
Cardano M, Diaferia GR, Falavigna M, Spinelli CC, Sessa F, DeBlasio P, Biunno I. Cardano M, et al. J Histochem Cytochem. 2013 Feb;61(2):116-24. doi: 10.1369/0022155412470455. Epub 2012 Nov 19. J Histochem Cytochem. 2013. PMID: 23172795 Free PMC article. - Protein Expression Analysis in Uterine Cervical Cancer for Potential Targets in Treatment.
Blancas S, Medina-Berlanga R, Ortíz-García L, Loredo-Ramírez A, Santos L. Blancas S, et al. Pathol Oncol Res. 2019 Apr;25(2):493-501. doi: 10.1007/s12253-018-0401-0. Epub 2018 Mar 12. Pathol Oncol Res. 2019. PMID: 29532409
References
- Ma J, Stampfer MJ, Giovannucci E, et al. Methylenetetrahydrofolate reductase polymorphism, dietary interactions, and risk of colorectal cancer. Cancer Res. 1997;57:1098–1102. - PubMed
- Ma Y, Hendershot LM. ER chaperone functions during normal and stress conditions. J Chem Neuroanat. 2004;28:51–65. - PubMed
- Orlowski RZ, Zeger EL. Targeting the proteasome as a therapeutic strategy against haematological malignancies. Expert Opin Investig Drugs. 2006;15:117–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA090890/CA/NCI NIH HHS/United States
- G12 RR003048/RR/NCRR NIH HHS/United States
- CA90890/CA/NCI NIH HHS/United States
- G12 RR003048-23/RR/NCRR NIH HHS/United States
- R01 CA102681/CA/NCI NIH HHS/United States
- CA102681/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical