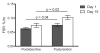Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein - PubMed (original) (raw)
Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein
Shanon L Casperson et al. Clin Nutr. 2012 Aug.
Abstract
Background & aim: Protein-energy supplementation is routinely employed to combat muscle loss. However, success is often compromised by increased satiety, poor palatability, high costs and low compliance.
Methods: For 2-weeks we supplemented meals of older individuals with leucine (4 g/meal; 3 meals/day; days 2-14). Metabolic studies were performed prior to (Day 1) and following (Day 15) supplementation. Leucine was not provided on metabolic study days. Venous blood and vastus lateralis muscle biopsies were obtained during a primed constant infusion of L-[ring-(13)C(6)] phenylalanine. Mixed muscle fractional synthesis rate (FSR), body composition and markers of nutrient signaling (mTOR, 4E-BP1 and p70S6K1 phosphorylation) were measured before and after a low protein/carbohydrate simulated meal.
Results: The meal modestly increased FSR on Day 1 (postabsorptive: 0.063 ± 0.004 vs. postprandial: 0.075 ± 0.006%/h; p = 0.03), however, two weeks of leucine supplementation increased postabsorptive FSR (p = 0.004) and the response to the meal (p = 0.01) (postabsorptive: 0.074 ± 0.007 vs. postprandial: 0.10 ± 0.007%/h). Changes in FSR were mirrored by increased phosphorylation of mTOR, 4E-BP1 and p70S6K1 (p ≤ 0.1). No change in fat free mass was observed (p > 0.05).
Conclusions: In older adults, leucine supplementation may improve muscle protein synthesis in response to lower protein meals.
Copyright © 2012 Elsevier Ltd and European Society for Clinical Nutrition and Metabolism. All rights reserved.
Figures
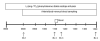
Fig. 1. Stable isotope infusion protocol
Stable isotope infusion studies were performed prior to (Day 1) and following (Day 15) leucine supplementation. Arterialized blood samples were obtained at 20-min intervals during an infusion of L-[ring-13C6] phenylalanine. Basal muscle biopsies from the vastus lateralis were obtained at 120 and 240 min. Postprandial muscle biopsies were obtained 30 and 180 min after consuming a simulated meal containing 7 g EAA and 10 g sugar.
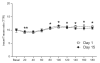
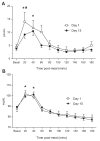
Fig. 2. Plasma phenylalanine enrichments
Plasma L-[ring-13C6] phenylalanine enrichments before and after the simulated meal on Day 1 and Day 15. Values are expressed as means ± SEM. * Significant difference from postabsorptive values.
Fig. 3. Mixed muscle fractional synthesis rate (FSR)
Comparison of the mixed muscle FSR measured before (black bars) and after (white bars) ingestion of the simulated meal on Day 1and again on Day 15. Values are expressed as means ± SEM.
Fig. 4. Intercellular and plasma mixed muscle fractional synthesis rate (FSR)
Comparison of the mixed-muscle postabsorptive (A) and postprandial (B) FSR measured on Day 1 and again on Day 15 utilizing muscle free phenylalanine as the precursor pool. Comparison of the mixed-muscle postabsorptive (C) and postprandial (D) FSR measured on Day 1 and again on Day 15 utilizing plasma phenylalanine as the precursor pool. Individual values are presented. Group values are expressed as means ± SEM.
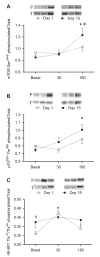
Fig. 5. Effects of chronic leucine supplementation on markers of nutrient signaling
Comparisons of the phosphorylation state of mTOR (A), p70S6K (B), and 4E-BP1 (C) obtained on Day 1 (□) and Day 15 (•). Protein phosphorylation and total protein were determined before and again at 30 and 180 min after ingestion of the simulated meal. A representative blot of the phosphorylation on Day 1 and Day 15 is shown above each graph. Values are expressed as means ± SEM. * significantly different from basal and # significantly different from Day 1; P < 0.05.
Fig. 6. Insulin and glucose concentrations
Plasma insulin (A) and glucose (B) concentrations obtained at Day 0 (□) and Day 14 (•) of leucine supplementation. Measurements were made before and every 20 min after ingestion of the simulated meal. Values are expressed as means ± SEM. * significantly different from basal and # significantly different from Day 1; P < 0.05.
Similar articles
- Protein-leucine fed dose effects on muscle protein synthesis after endurance exercise.
Rowlands DS, Nelson AR, Phillips SM, Faulkner JA, Clarke J, Burd NA, Moore D, Stellingwerff T. Rowlands DS, et al. Med Sci Sports Exerc. 2015 Mar;47(3):547-55. doi: 10.1249/MSS.0000000000000447. Med Sci Sports Exerc. 2015. PMID: 25026454 Clinical Trial. - Leucine supplementation enhances integrative myofibrillar protein synthesis in free-living older men consuming lower- and higher-protein diets: a parallel-group crossover study.
Murphy CH, Saddler NI, Devries MC, McGlory C, Baker SK, Phillips SM. Murphy CH, et al. Am J Clin Nutr. 2016 Dec;104(6):1594-1606. doi: 10.3945/ajcn.116.136424. Epub 2016 Nov 9. Am J Clin Nutr. 2016. PMID: 27935521 Clinical Trial. - Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia.
Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, Mosoni L, Dardevet D. Rieu I, et al. J Physiol. 2006 Aug 15;575(Pt 1):305-15. doi: 10.1113/jphysiol.2006.110742. Epub 2006 Jun 15. J Physiol. 2006. PMID: 16777941 Free PMC article. Clinical Trial. - International society of sports nutrition position stand: nutrient timing.
Kerksick CM, Arent S, Schoenfeld BJ, Stout JR, Campbell B, Wilborn CD, Taylor L, Kalman D, Smith-Ryan AE, Kreider RB, Willoughby D, Arciero PJ, VanDusseldorp TA, Ormsbee MJ, Wildman R, Greenwood M, Ziegenfuss TN, Aragon AA, Antonio J. Kerksick CM, et al. J Int Soc Sports Nutr. 2017 Aug 29;14:33. doi: 10.1186/s12970-017-0189-4. eCollection 2017. J Int Soc Sports Nutr. 2017. PMID: 28919842 Free PMC article. Review. - Protecting Skeletal Muscle with Protein and Amino Acid during Periods of Disuse.
Galvan E, Arentson-Lantz E, Lamon S, Paddon-Jones D. Galvan E, et al. Nutrients. 2016 Jul 1;8(7):404. doi: 10.3390/nu8070404. Nutrients. 2016. PMID: 27376322 Free PMC article. Review.
Cited by
- Effects of an Oral Nutritional Supplementation and Physical Exercise Intervention on Older Adults at Risk for Sarcopenia.
Gonçalves TJM, Carlos BT, de Souza MS, Jorge VC, Gonçalves SEAB, Campos RA, Rosenfeld VAS. Gonçalves TJM, et al. J Frailty Sarcopenia Falls. 2024 Sep 1;9(3):184-191. doi: 10.22540/JFSF-09-184. eCollection 2024 Sep. J Frailty Sarcopenia Falls. 2024. PMID: 39228667 Free PMC article. - Untargeted metabolomic profiling of serum from client-owned cats with early and late-stage chronic kidney disease.
Nealon NJ, Summers S, Quimby J, Winston JA. Nealon NJ, et al. Sci Rep. 2024 Feb 27;14(1):4755. doi: 10.1038/s41598-024-55249-5. Sci Rep. 2024. PMID: 38413739 Free PMC article. - Loss of Muscle Mass and Strength After Hip Fracture: an Intervention Target for Nutrition Supplementation.
Reider L, Owen EC, Dreyer HC, Fitton LS, Willey MC; METRC (Major Extremity Trauma Research Consortium). Reider L, et al. Curr Osteoporos Rep. 2023 Dec;21(6):710-718. doi: 10.1007/s11914-023-00836-0. Epub 2023 Nov 29. Curr Osteoporos Rep. 2023. PMID: 38019345 Review. - Amino acid-stimulated insulin secretion: a path forward in type 2 diabetes.
Kolic J, Sun WG, Johnson JD, Guess N. Kolic J, et al. Amino Acids. 2023 Dec;55(12):1857-1866. doi: 10.1007/s00726-023-03352-8. Epub 2023 Nov 15. Amino Acids. 2023. PMID: 37966501 Review. - Nutritional Interventions: Dietary Protein Needs and Influences on Skeletal Muscle of Older Adults.
Campbell WW, Deutz NEP, Volpi E, Apovian CM. Campbell WW, et al. J Gerontol A Biol Sci Med Sci. 2023 Jun 16;78(Suppl 1):67-72. doi: 10.1093/gerona/glad038. J Gerontol A Biol Sci Med Sci. 2023. PMID: 37325954 Free PMC article. Review.
References
- Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82(5):1065–73. - PubMed
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. Faseb J. 2005;19(3):422–4. - PubMed
- Paddon-Jones D, Sheffield-Moore M, Aarsland A, Wolfe RR, Ferrando AA. Exogenous amino acids stimulate human muscle anabolism without interfering with the response to mixed meal ingestion. Am J Physiol Endocrinol Metab. 2005;288(4):E761–7. - PubMed
- Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR. Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr. 2008;87(5):1562S–6S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 HD007539/HD/NICHD NIH HHS/United States
- T32HD007539/HD/NICHD NIH HHS/United States
- R01 CA127971/CA/NCI NIH HHS/United States
- UL1 TR000071/TR/NCATS NIH HHS/United States
- 1UL1RR029876/RR/NCRR NIH HHS/United States
- UL1 RR029876/RR/NCRR NIH HHS/United States
- 5R01 CA127971/CA/NCI NIH HHS/United States
- P30 AG024832/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous