Transcriptional silencing of the Wnt-antagonist DKK1 by promoter methylation is associated with enhanced Wnt signaling in advanced multiple myeloma - PubMed (original) (raw)
Transcriptional silencing of the Wnt-antagonist DKK1 by promoter methylation is associated with enhanced Wnt signaling in advanced multiple myeloma
Kinga A Kocemba et al. PLoS One. 2012.
Abstract
The Wnt/β-catenin pathway plays a crucial role in the pathogenesis of various human cancers. In multiple myeloma (MM), aberrant auto-and/or paracrine activation of canonical Wnt signaling promotes proliferation and dissemination, while overexpression of the Wnt inhibitor Dickkopf1 (DKK1) by MM cells contributes to osteolytic bone disease by inhibiting osteoblast differentiation. Since DKK1 itself is a target of TCF/β-catenin mediated transcription, these findings suggest that DKK1 is part of a negative feedback loop in MM and may act as a tumor suppressor. In line with this hypothesis, we show here that DKK1 expression is low or undetectable in a subset of patients with advanced MM as well as in MM cell lines. This absence of DKK1 is correlated with enhanced Wnt pathway activation, evidenced by nuclear accumulation of β-catenin, which in turn can be antagonized by restoring DKK1 expression. Analysis of the DKK1 promoter revealed CpG island methylation in several MM cell lines as well as in MM cells from patients with advanced MM. Moreover, demethylation of the DKK1 promoter restores DKK1 expression, which results in inhibition of β-catenin/TCF-mediated gene transcription in MM lines. Taken together, our data identify aberrant methylation of the DKK1 promoter as a cause of DKK1 silencing in advanced stage MM, which may play an important role in the progression of MM by unleashing Wnt signaling.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
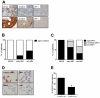
Figure 1. The relation between the Wnt pathway activation and the loss of DKK1 expression during MM progression.
(A) Representative pictures of immunohistochemical staining of a multiple myeloma patient displaying either high DKK-1 and low β-catenin expression (upper panel) or with DKK-1 loss and increased nuclear β-catenin localization (lower panel). Immunohistochemical stainings are shown for CD138 (left column), β-catenin (middle column) and DKK1 (right column). (B) Nuclear β-catenin expression in relation to multiple myeloma progression (n = 48, p<0.05). (C) DKK-1 expression in relation to multiple myeloma progression (p<0.05). (D) Representative pictures of immunocytochemical staining of multiple myeloma cell lines with goat polyclonal anti-DKK1 antibody (magnification: 400×). Prostate cancer cell line (PC-3) was used as positive control (PC) for the DKK-1 staining. (E) Relation between the loss of DKK-1 expression and nuclear localization of β-catenin (p>0.05). A significant correlation (p<0.05) between expression of nuclear β-catenin and DKK-1 was observed in the two extreme groups identified based on β-catenin expression. * indicates p value<0.05.
Figure 2. DKK1 expression represses Wnt pathway activation in MM.
(A) MM cell lines OPM-1 and UM-1 were transduced with either the LZRS-pBMN-IRES-eGFP (control) or the LZRS-pBMN-DKK1-IRES-eGFP (DKK1) virus. Conditioned medium of sorted, transduced cells was harvested and immunoblotted using a goat polyclonal antibody against DKK1. Representative immunoblot confirms the expression of DKK1 in the conditioned medium of LZRS-pBMN-DKK1-IRES-eGFP transduced cells. β-actin is shown as internal control for equal cell number. (B) Cytoplasmic and nuclear proteins were prepared from the LZRS-pBMN-IRES-eGFP (control) or the LZRS-pBMN-DKK1-IRES-eGFP (DKK1) MM cells, stimulated for 24h with Wnt3a conditioned medium (+). As a control, L-cells conditioned medium was applied (−).To assess β-catenin accumulation, nuclear and cytoplasmic cells lysate was immunoblotted by using a monoclonal anti-β-catenin antibody. The bottom part of the blot was stained with β-tubulin and Histone H2B as controls for cytoplasmic and nuclear proteins, respectively. (C) LZRS-pBMN-IRES-eGFP (control) or the LZRS-pBMN-DKK1-IRES-eGFP (DKK1) cells were transfected with TOPFLASH reporter and renilla contruct. 24 hours upon transfection cells were treated with L-cells conditioned medium (−) or Wnt3a conditioned medium (+).The relative light units value of LZRS-pBMN-IRES-eGFP cells treated with L-cells conditioned medium was normalized to 1. The mean ± SD of representative experiment performed in triplicate is shown. * indicates p value<0.05 *** indicates p value<0.001. by student's t test.
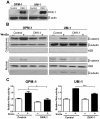
Figure 3. DKK1 promoter methylation in MM cell lines.
(A) Total RNA was isolated and RT–PCR analyses were performed with the specific primers indicated. Complementary DNA from prostate cancer cell line (PC-3) was used as positive control (PC) for DKK1 expression. The β-actin expression was used as a loading control. (B) Schematic representation of the promoter area analyzed for DKK1, containing a CpG island. White arrows indicate the positions of primers used for bisulfite sequencing, and black arrows indicate the positions of primers used for methylation specific PCR. Each of the CpG dinucleotides is presented as open circle. (C) Upper panel. Representation of bisulfite genomic sequencing results of 5 clones of the DKK1 promoter region in HT-29 and DLD-1 colon cell lines used as unmethylated (U_DNA) and methylated (M_DNA) control, respectively. The amplified 326 bp product corresponds to the DKK1 promoter region from −193 to +122. In total, 18 CpG dinucleotides (CpGs) within the CpG island were analyzed and are represented as open and closed circles, which indicate unmethylated and methylated CpG sites, respectively. Lower panel. Electropherograms of bisulfite modified DNA from DKK1 CpG island in HT-29 (U_DNA) and DLD-1 (M_DNA) cells. (D) Methylation specific PCR of the CpG island of the DKK1 promoter region in MM cell lines. DNA bands in lanes labeled with U indicate PCR products amplified with primers recognizing unmethylated promoter sequences, whereas DNA bands in lanes labeled with M represent amplified products with primers designed for the methylated template. (E) Bisulfite sequencing analysis of the the DKK1 promoter region in multiple myeloma cell lines, open circles indicating unmethylated CpG sites, and closed circles representing methylated CpG sites. Percentages indicate the fraction of methylated CpG dinucleotides of the total CpG sites analyzed.
Figure 4. Analysis of DKK1 promoter methylation in MM bone marrow samples.
Methylation specific PCR of the CpG island of the DKK1 promoter region in the bone marrow samples of twelve patients with multiple myeloma (P1–P12), HT-29 and DLD-1 colon cell lines were used as unmethylated (U_DNA) and methylated (M_DNA) control respectively. DNA bands in lanes labeled with U and M indicate PCR products amplified with primers recognizing unmethylated and methylated promoter sequences respectively.
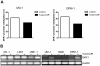
Figure 5. Restoration of DKK1 expression in MM cell lines by 5-aza-2-deoxycytidine treatment.
(A) DKK-1 promoter methylation analyzed by bisulfite genomic sequencing of 10 clones, on DNA isolated from 5-aza-2-deoxycytidine treated (5-aza-CdR) and untreated (PBS) MM cell lines UM-1 and OPM-1. Frequency of methylation was calculated by dividing the number of methylated CpG sites by the total number of analyzed CpG sites. (B) Reverse transcriptase-PCR analysis for DKK1 gene expression in multiple myeloma cell lines in the absence and presence of the demethylating agent 5-aza-2-deoxycytidine. β-actin expression is shown as an input control.
Similar articles
- Dkk1-induced inhibition of Wnt signaling in osteoblast differentiation is an underlying mechanism of bone loss in multiple myeloma.
Qiang YW, Barlogie B, Rudikoff S, Shaughnessy JD Jr. Qiang YW, et al. Bone. 2008 Apr;42(4):669-80. doi: 10.1016/j.bone.2007.12.006. Epub 2007 Dec 27. Bone. 2008. PMID: 18294945 - The Wnt antagonists DKK1 and SFRP1 are downregulated by promoter hypermethylation in systemic sclerosis.
Dees C, Schlottmann I, Funke R, Distler A, Palumbo-Zerr K, Zerr P, Lin NY, Beyer C, Distler O, Schett G, Distler JH. Dees C, et al. Ann Rheum Dis. 2014 Jun;73(6):1232-9. doi: 10.1136/annrheumdis-2012-203194. Epub 2013 May 22. Ann Rheum Dis. 2014. PMID: 23698475 - Aberrant Wnt signaling in multiple myeloma: molecular mechanisms and targeting options.
van Andel H, Kocemba KA, Spaargaren M, Pals ST. van Andel H, et al. Leukemia. 2019 May;33(5):1063-1075. doi: 10.1038/s41375-019-0404-1. Epub 2019 Feb 15. Leukemia. 2019. PMID: 30770859 Free PMC article. Review. - Dickkopf-1 is a key regulator of myeloma bone disease: opportunities and challenges for therapeutic intervention.
Zhou F, Meng S, Song H, Claret FX. Zhou F, et al. Blood Rev. 2013 Nov;27(6):261-7. doi: 10.1016/j.blre.2013.08.002. Epub 2013 Sep 2. Blood Rev. 2013. PMID: 24054128 Free PMC article. Review.
Cited by
- EBV miR-BART10-3p Promotes Cell Proliferation and Migration by Targeting DKK1.
Min K, Lee SK. Min K, et al. Int J Biol Sci. 2019 Jan 24;15(3):657-667. doi: 10.7150/ijbs.30099. eCollection 2019. Int J Biol Sci. 2019. PMID: 30745852 Free PMC article. - Cancer Metastases to Bone: Concepts, Mechanisms, and Interactions with Bone Osteoblasts.
Shupp AB, Kolb AD, Mukhopadhyay D, Bussard KM. Shupp AB, et al. Cancers (Basel). 2018 Jun 4;10(6):182. doi: 10.3390/cancers10060182. Cancers (Basel). 2018. PMID: 29867053 Free PMC article. Review. - Aberrant DNA Methylation Profile of Dickkopf-1 in Ankylosing Spondylitis.
Sun X, Deng Y, Ni M, Zhang T, Wang X, Wu Y, Shuai Z, Pan F. Sun X, et al. Biochem Genet. 2024 Dec;62(6):4603-4618. doi: 10.1007/s10528-024-10675-y. Epub 2024 Feb 12. Biochem Genet. 2024. PMID: 38347292 - Galectin-3 accelerates the progression of oral tongue squamous cell carcinoma via a Wnt/β-catenin-dependent pathway.
Wang LP, Chen SW, Zhuang SM, Li H, Song M. Wang LP, et al. Pathol Oncol Res. 2013 Jul;19(3):461-74. doi: 10.1007/s12253-013-9603-7. Epub 2013 Mar 22. Pathol Oncol Res. 2013. PMID: 23519607 - Wnt signaling in multiple myeloma: a central player in disease with therapeutic potential.
Spaan I, Raymakers RA, van de Stolpe A, Peperzak V. Spaan I, et al. J Hematol Oncol. 2018 May 18;11(1):67. doi: 10.1186/s13045-018-0615-3. J Hematol Oncol. 2018. PMID: 29776381 Free PMC article. Review.
References
- Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374:324–329. - PubMed
- Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Donofrio G, et al. Production of Wnt inhibitors by myeloma cells: potential effects on canonical Wnt pathway in the bone microenvironment. Cancer Res. 2007;67:7665–7674. - PubMed
- Oshima T, Abe M, Asano J, Hara T, Kitazoe K, et al. Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood. 2005;106:3160–3165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical