AMPK: a nutrient and energy sensor that maintains energy homeostasis - PubMed (original) (raw)
Review
AMPK: a nutrient and energy sensor that maintains energy homeostasis
D Grahame Hardie et al. Nat Rev Mol Cell Biol. 2012.
Abstract
AMP-activated protein kinase (AMPK) is a crucial cellular energy sensor. Once activated by falling energy status, it promotes ATP production by increasing the activity or expression of proteins involved in catabolism while conserving ATP by switching off biosynthetic pathways. AMPK also regulates metabolic energy balance at the whole-body level. For example, it mediates the effects of agents acting on the hypothalamus that promote feeding and entrains circadian rhythms of metabolism and feeding behaviour. Finally, recent studies reveal that AMPK conserves ATP levels through the regulation of processes other than metabolism, such as the cell cycle and neuronal membrane excitability.
Figures
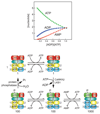
Figure 1
Model for the mechanism by which AMPK is activated by increases in AMP and ADP (top), as the cellular concentrations of ATP, ADP and AMP change (bottom). The model at the top represents different states of the three subunits of AMPK (see Fig. 2 for details of domains). The graph at the bottom shows changes in the predicted concentrations of ATP, ADP and AMP on going from an unstressed, fully charged cell (left) to a cell undergoing a severe energy stress (right), corresponding to a 10-fold increase in ADP:ATP ratio. The graph was generated by assuming that the adenylate kinase reaction was at equilibrium. Note that in a fully charged cell (left), AMP concentration is very low, but that its % change in concentration as ADP/ATP increases is always much greater than those of ATP or ADP. In the model at the top, the basal state (top left) has sites 1 and 3 in the γ subunit occupied by ATP (site 4 is always occupied by AMP). Replacement of ATP by ADP (or AMP) at site 3 during moderate stress (top centre) promotes phosphorylation of Thr172 (bottom centre), causing a 100-fold increase in activity (indicated by two stars). Replacement of ATP by AMP at site 1 during more severe stress causes a further 10-fold allosteric activation (indicated by a third star, bottom right). As cellular energy status returns to normal, AMP at site 1 and ADP or AMP at site 3 are progressively replaced by ATP (moving from right to left on the bottom row). This promotes the dephosphorylation of Thr-172 and a return to the basal state.
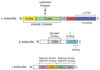
Figure 2
Domain map of typical mammalian AMPK. Colour coding of domains are similar to those in Figs. 1 and 3. AMPK complexes are heterotrimers composed of α, β and γ subunits in a 1:1:1 ratio. Key to acronyms: N-lobe, N-terminal lobe of kinase domain; C-lobe, C-terminal lobe of kinase domain; AID, auto-inhibitory domain; α-CTD, α subunit C-terminal domain; CBM, carbohydrate-binding module; β-CTD, β subunit C-terminal domain; CBS1-4, CBS repeats in γ subunit. The β-CTD forms the core of the complex, binding to the α-CTD and the N-terminus of the γ subunit prior to CBS1.
Figure 3
Two views of a crystal structure of a partial heterotrimeric complex of mammalian AMPK; the right-hand view is rotated about 180° about the y axis compared to the left-hand view. The constructs crystallized contained only the C-terminal domain of the β subunit and also lacked a flexible loop in the C-terminal domain of the α subunit. The α subunit AID was present but was not resolved in this crystal form; in the left-hand view, its approximate location would just to the right of the junction between the αsubunit C-lobe (green) and the α-linker (red). The crystals contained AMP bound at sites 3 and 4. Note how in this structure, access to the Thr172 site is restricted by the close association of the α-CTD with the kinase domain (left-hand view). In addition, note how AMP bound in site 3 is not visible because it is covered by the “α-hook” structure of the linker peptide (right-hand view, linker peptide in red). Key to acronyms: N-lobe, N-terminal lobe of kinase domain; C-lobe, C-terminal lobe of kinase domain; γ-NTD, N-terminal region of γ subunit; α-CTD, α subunit C-terminal domain; β-CTD, β subunit C-terminal domain; γ-CBS1-4, CBS repeats in γ subunit.
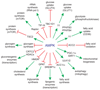
Figure 4
Summary of effects of AMPK activation on cellular metabolism. The likely protein targets that mediate the metabolic effects of AMPK, as well as the final metabolic outcomes, are depicted. Proteins shown on the inner wheel with question marks may not be directly phosphorylated by AMPK. Catabolic pathways, including glucose uptake via GLUT4 and GLUT1, glycolysis, fatty acid uptake via CD36, fatty acid oxidation, mitochondrial biogenesis and autophagy are invariably activated by AMPK. Anabolic pathways, including fatty acid synthesis, transcription of lipogenic enzymes, triglyceride synthesis, cholesterol synthesis, transcription of gluconeogenic enzymes, glycogen synthesis, protein synthesis and rRNA synthesis, are invariably inhibited by AMPK.
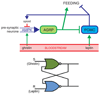
Figure 5
AMPK-regulated control of feeding behavior by modulation of neuropeptide Y-and agouti-related protein-expressing neurons (NPY/AgRP neurons) and pro-opiomelanocortin neurons (POMC neurons), as proposed by Yang et al. A) in the fasted state ghrelin, a “hunger signal” derived from the stomach, activates AMPK in the presynaptic neurons acting upstream of NPY/AgRP neurons via the CaMKKβ pathway. This causes release of Ca2+ by Ca2+-induced release from intercellular stores via ryanodine receptors, creating a feedback loop that causes continued release of neurotransmitter onto the NPY/AgRP neuron, even when ghrelin stimulation ceases. The NPY/AgRP neurons promote feeding (and inhibits the POMC neurons, which inhibit feeding). b) Feeding continues (even in the absence of ghrelin) until the POMC neurons are stimulated by the “satiety signal”, leptin. Activity of these neurons inhibits feeding and also promotes release of opioids that inhibit AMPK in the presynaptic neurons upstream of the NPY/AgRP neurons, switching them back to an inactive state.
Similar articles
- Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism.
Ke R, Xu Q, Li C, Luo L, Huang D. Ke R, et al. Cell Biol Int. 2018 Apr;42(4):384-392. doi: 10.1002/cbin.10915. Epub 2018 Jan 3. Cell Biol Int. 2018. PMID: 29205673 Review. - AMPK: positive and negative regulation, and its role in whole-body energy homeostasis.
Hardie DG. Hardie DG. Curr Opin Cell Biol. 2015 Apr;33:1-7. doi: 10.1016/j.ceb.2014.09.004. Epub 2014 Sep 26. Curr Opin Cell Biol. 2015. PMID: 25259783 Review. - [Regulation of energy metabolism by AMPK: a novel therapeutic approach for the treatment of metabolic and cardiovascular diseases].
Foretz M, Taleux N, Guigas B, Horman S, Beauloye C, Andreelli F, Bertrand L, Viollet B. Foretz M, et al. Med Sci (Paris). 2006 Apr;22(4):381-8. doi: 10.1051/medsci/2006224381. Med Sci (Paris). 2006. PMID: 16597407 Review. French. - Allosteric regulation of AMP-activated protein kinase by adenylate nucleotides and small-molecule drugs.
de Souza Almeida Matos AL, Oakhill JS, Moreira J, Loh K, Galic S, Scott JW. de Souza Almeida Matos AL, et al. Biochem Soc Trans. 2019 Apr 30;47(2):733-741. doi: 10.1042/BST20180625. Epub 2019 Apr 18. Biochem Soc Trans. 2019. PMID: 31000529 Review. - AMP-activated protein kinase: also regulated by ADP?
Hardie DG, Carling D, Gamblin SJ. Hardie DG, et al. Trends Biochem Sci. 2011 Sep;36(9):470-7. doi: 10.1016/j.tibs.2011.06.004. Epub 2011 Jul 23. Trends Biochem Sci. 2011. PMID: 21782450 Review.
Cited by
- Rab2A-mediated Golgi-lipid droplet interactions support very-low-density lipoprotein secretion in hepatocytes.
Xu M, Chen ZY, Li Y, Li Y, Guo G, Dai RZ, Ni N, Tao J, Wang HY, Chen QL, Wang H, Zhou H, Yang YN, Chen S, Chen L. Xu M, et al. EMBO J. 2024 Dec;43(24):6383-6409. doi: 10.1038/s44318-024-00288-x. Epub 2024 Nov 4. EMBO J. 2024. PMID: 39496977 Free PMC article. - Metabolic Pathophysiology of Cortical Spreading Depression: A Review.
Hill A, Amendolara AB, Small C, Guzman SC, Pfister D, McFarland K, Settelmayer M, Baker S, Donnelly S, Payne A, Sant D, Kriak J, Bills KB. Hill A, et al. Brain Sci. 2024 Oct 16;14(10):1026. doi: 10.3390/brainsci14101026. Brain Sci. 2024. PMID: 39452037 Free PMC article. Review. - Impaired oxidative phosphorylation regulates necroptosis in human lung epithelial cells.
Koo MJ, Rooney KT, Choi ME, Ryter SW, Choi AM, Moon JS. Koo MJ, et al. Biochem Biophys Res Commun. 2015 Aug 28;464(3):875-80. doi: 10.1016/j.bbrc.2015.07.054. Epub 2015 Jul 14. Biochem Biophys Res Commun. 2015. PMID: 26187663 Free PMC article. - Sphingolipids are required for efficient triacylglycerol loss in conjugated linoleic Acid treated adipocytes.
Wang W, Fromm M. Wang W, et al. PLoS One. 2015 Apr 23;10(4):e0119005. doi: 10.1371/journal.pone.0119005. eCollection 2015. PLoS One. 2015. PMID: 25906159 Free PMC article. - An AMP-activated protein kinase complex with two distinctive alpha subunits is involved in nutritional stress responses in Trypanosoma cruzi.
Sternlieb T, Schoijet AC, Genta PD, Vilchez Larrea SC, Alonso GD. Sternlieb T, et al. PLoS Negl Trop Dis. 2021 May 24;15(5):e0009435. doi: 10.1371/journal.pntd.0009435. eCollection 2021 May. PLoS Negl Trop Dis. 2021. PMID: 34029334 Free PMC article.
References
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. - PubMed
- Suter M, et al. Dissecting the role of 5'-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem. 2006;281:32207–32216. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources