BRCA1 functions independently of homologous recombination in DNA interstrand crosslink repair - PubMed (original) (raw)
. 2012 Apr 27;46(2):125-35.
doi: 10.1016/j.molcel.2012.02.015. Epub 2012 Mar 22.
Elsa Callén, Marina L Kozak, Jung Min Kim, Nancy Wong, Andrés J López-Contreras, Thomas Ludwig, Richard Baer, Robert B Faryabi, Amy Malhowski, Hua-Tang Chen, Oscar Fernandez-Capetillo, Alan D'Andrea, André Nussenzweig
Affiliations
- PMID: 22445484
- PMCID: PMC3340543
- DOI: 10.1016/j.molcel.2012.02.015
BRCA1 functions independently of homologous recombination in DNA interstrand crosslink repair
Samuel F Bunting et al. Mol Cell. 2012.
Abstract
Brca1 is required for DNA repair by homologous recombination (HR) and normal embryonic development. Here we report that deletion of the DNA damage response factor 53BP1 overcomes embryonic lethality in Brca1-nullizygous mice and rescues HR deficiency, as measured by hypersensitivity to polyADP-ribose polymerase (PARP) inhibition. However, Brca1,53BP1 double-deficient cells are hypersensitive to DNA interstrand crosslinks (ICLs), indicating that BRCA1 has an additional role in DNA crosslink repair that is distinct from HR. Disruption of the nonhomologous end-joining (NHEJ) factor, Ku, promotes DNA repair in Brca1-deficient cells; however deletion of either Ku or 53BP1 exacerbates genomic instability in cells lacking FANCD2, a mediator of the Fanconi anemia pathway for ICL repair. BRCA1 therefore has two separate roles in ICL repair that can be modulated by manipulating NHEJ, whereas FANCD2 provides a key activity that cannot be bypassed by ablation of 53BP1 or Ku.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
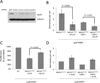
Figure 1. Ku expression correlates with genomic instability and reduced proliferation in _Brca1_-deficient cells treated with PARP inhibitor
(A) Ku70 expression in cells expressing Ku70 shRNAs. First lane (GFP) shows expression of Ku70 in cells expressing a control shRNA specific for GFP. The GFP and KU70.1 shRNAs were selected for integration into Brca1Δ11/Δ11 MEFs. (B) Average genomic instability observed in metaphase spreads from MEF cells treated overnight with 1µM PARP inhibitor (C) Proliferation of MEFs growing in the presence of 1µM PARP inhibitor, relative to untreated cells. (D) Genomic instability in metaphases from MEFs overexpressing rat Ku70. Error bars show standard deviation in each case. See also Figure S1.
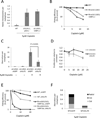
Figure 2. Reduced genomic instability and increased survival of _Brca1_-deficient cells with Ku70 knockdown after cisplatin treatment
(A) Frequency of radial chromosome formation in lymphocytes of the indicated genotypes after overnight treatment with 5µM cisplatin. (B) Colony formation in MEFs treated for 2 hrs with cisplatin. (C) Genomic instability in MEFs expressing stably integrated shRNA against either GFP or Ku70, treated overnight with 5µM cisplatin. (D) Growth of BRCA1Δ11/Δ11 cells with stably integrated Ku70 shRNA in the presence of cisplatin. Cisplatin was applied for 24 hrs and growth assayed with CellTiter-Glo after a total 5 days. (E) Colony formation of MEF lines treated for 2hrs with cisplatin. (F) Genomic instability in control (WTshGFP) or Ku70 knockdown MEFs after overnight treatment with 5µM cisplatin. Error bars show standard deviation in each case. See also Figure S2.
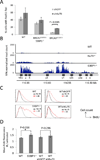
Figure 3. Effect of 53BP1 and Ku on Rad51 foci and DSB resection
(A) Quantification of Rad51 immunofluorescence in mouse embryonic fibroblasts of the indicated genotypes. Cell expressing either control shRNA against GFP or shRNA against Ku70 were irradiated (5Gy, 4hrs recovery) and stained with anti-Rad51 antibody. The average percentages of cells (+/− standard deviation) with more than 5 nuclear foci from three experiments are shown. (B) Anti-RPA ChIP-Seq in B cells. B cells were stimulated to undergo class switch recombination in vitro. Chromatin from B cells was harvested 48 hrs post-stimulation and used for RPA ChIP. RPA read count (normalized by the total library size per million) is shown at the IgH locus. (C) Non-denaturing anti-BrdU immunofluorescence in MEFs treated with ionizing radiation (30Gy, 2 hrs recovery), measured by flow cytometry. Resection is measured by detection of exposed BrdU (x-axis). (D) Quantification of mean BrdU fluorescence intensity of the irradiated population shown in (C), normalized to the untreated population. Average +/− standard deviation from 5 experiments. See also Figure S4.
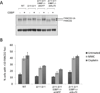
Figure 4. FANCD2 ubiquitylation and damage foci in _Brca1_-deficient cells
(A) Western Blot showing FANCD2 ubiquitylation in WT, Brca1Δ11/Δ11 and Brca1Δ11/Δ1153BP1−/− MEFs with and without cisplatin (CDDP) treatment. Brca1Δ11/Δ1153BP1−/− MEFs expressed either control shRNA or shRNA against Ku70. (B) FANCD2 foci analysis in MEFs treated with either cisplatin or mitomycin C. Cells with more than 10 FANCD2 foci were scored as positive. Mean +/− SEM shown. See also Figure S3.
Figure 5. Genomic instability in metaphases from cells treated overnight with drugs to induce DNA interstrand cross-links (ICLs)
(A) Genomic instability in B cells from WT, FANCD2−/− and FANCD2−/−53BP1−/− mice treated overnight with 5µM cisplatin. (B) Genomic instability in B cells from WT, FANCD2−/− and FANCD2−/−53BP1−/− mice treated overnight with 250 nM mitomyin C (MMC). (C) Total genomic aberrations in metaphases from FANCD2−/− and FANCD2−/− Ku80−/− mouse embryonic fibroblast cells after overnight treatment with 5µM cisplatin. (D) Genomic instability in metaphases from FANCD2−/− and FANCD2−/−Ku80−/− after overnight treatment with 250nM MMC. (E) Colony forming assay in WT, FANCD2−/− and FANCD2−/−Ku80−/− MEFs treated with cisplatin. (F) Colony forming assay WT, FANCD2−/− and FANCD2,Ku80 double-knockout MEFs treated with mitomycin C. (Mean +/− standard deviation shown.) See also Figure S5.
Figure 6. Model for repair of DNA double-strand breaks (DSBs) induced by PARP inhibitor or DNA cross-linking agents
(a) Treatment with PARP inhibitor stabilizes spontaneous DNA single-strand breaks, which are converted to DSBs during DNA replication (Bryant et al., 2005; Farmer et al., 2005). (b) DNA DSBs can be repaired either by NHEJ or HR. (c) 5′ – 3′ exonuclease resection commits repair to the error-free HR pathway. 53BP1 antagonizes double-strand break resection. (d) Ku70/80 can potentially join the DSB to a second DNA end present in the cells to cause chromosome rearrangements. (e) Treatment with cisplatin or MMC generates interstrand DNA cross-links. (f) Interstrand cross-links cause replication fork stalling and collapse. Accumulation of FANCI/D2, dependent on BRCA1, recruits endonucleases that cut DNA on either side of the interstrand cross-link to generate a double-strand break (g). (h) Trans-lesion synthesis (TLS) and (i) nucleotide excision repair (NER) re-generate duplex DNA on one sister chromatid, enabling homology-dependent repair of the DNA DSB. (j) Aberrant joining mediated by Ku70/80-competes with normal repair and can potentially generate chromosome rearrangements leading to cancer or cell death.
Comment in
- A novel function for BRCA1 in crosslink repair.
Long DT, Walter JC. Long DT, et al. Mol Cell. 2012 Apr 27;46(2):111-2. doi: 10.1016/j.molcel.2012.04.010. Mol Cell. 2012. PMID: 22541552 Free PMC article.
Similar articles
- Regulation of DNA repair in the absence of classical non-homologous end joining.
Kang YJ, Yan CT. Kang YJ, et al. DNA Repair (Amst). 2018 Aug;68:34-40. doi: 10.1016/j.dnarep.2018.06.001. Epub 2018 Jun 12. DNA Repair (Amst). 2018. PMID: 29929045 - ZMYM2 restricts 53BP1 at DNA double-strand breaks to favor BRCA1 loading and homologous recombination.
Lee D, Apelt K, Lee SO, Chan HR, Luijsterburg MS, Leung JWC, Miller KM. Lee D, et al. Nucleic Acids Res. 2022 Apr 22;50(7):3922-3943. doi: 10.1093/nar/gkac160. Nucleic Acids Res. 2022. PMID: 35253893 Free PMC article. - BRCA1 and HSP90 cooperate in homologous and non-homologous DNA double-strand-break repair and G2/M checkpoint activation.
Stecklein SR, Kumaraswamy E, Behbod F, Wang W, Chaguturu V, Harlan-Williams LM, Jensen RA. Stecklein SR, et al. Proc Natl Acad Sci U S A. 2012 Aug 21;109(34):13650-5. doi: 10.1073/pnas.1203326109. Epub 2012 Aug 6. Proc Natl Acad Sci U S A. 2012. PMID: 22869732 Free PMC article. - Beyond interstrand crosslinks repair: contribution of FANCD2 and other Fanconi Anemia proteins to the replication of DNA.
Federico MB, Campodónico P, Paviolo NS, Gottifredi V. Federico MB, et al. Mutat Res. 2018 Mar;808:83-92. doi: 10.1016/j.mrfmmm.2017.09.004. Epub 2017 Sep 14. Mutat Res. 2018. PMID: 29031493 Review. - Interplay between Fanconi anemia and homologous recombination pathways in genome integrity.
Michl J, Zimmer J, Tarsounas M. Michl J, et al. EMBO J. 2016 May 2;35(9):909-23. doi: 10.15252/embj.201693860. Epub 2016 Apr 1. EMBO J. 2016. PMID: 27037238 Free PMC article. Review.
Cited by
- Analysis for type of 53BP1 nuclear expression by immunofluorescence as an indicator of genomic instability in oropharyngeal squamous epithelial lesions.
Nishi H, Matsuda K, Terakado M, Kondo H, Kumai Y, Nakashima M. Nishi H, et al. Sci Rep. 2024 Nov 11;14(1):27525. doi: 10.1038/s41598-024-77945-y. Sci Rep. 2024. PMID: 39528680 Free PMC article. - How to sensitize glioblastomas to temozolomide chemotherapy: a gap-centered view.
Miramova A, Gartner A, Ivanov D. Miramova A, et al. Front Cell Dev Biol. 2024 Jul 1;12:1436563. doi: 10.3389/fcell.2024.1436563. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39011394 Free PMC article. Review. - E3 ligases: a ubiquitous link between DNA repair, DNA replication and human disease.
Chauhan AS, Jhujh SS, Stewart GS. Chauhan AS, et al. Biochem J. 2024 Jul 17;481(14):923-944. doi: 10.1042/BCJ20240124. Biochem J. 2024. PMID: 38985307 Free PMC article. Review. - Research progress on the fanconi anemia signaling pathway in non-obstructive azoospermia.
Xu H, Zhang Y, Wang C, Fu Z, Lv J, Yang Y, Zhang Z, Qi Y, Meng K, Yuan J, Wang X. Xu H, et al. Front Endocrinol (Lausanne). 2024 May 23;15:1393111. doi: 10.3389/fendo.2024.1393111. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38846492 Free PMC article. Review. - Brca1 Mouse Models: Functional Insights and Therapeutic Opportunities.
Yueh WT, Glass DJ, Johnson N. Yueh WT, et al. J Mol Biol. 2024 Jan 1;436(1):168372. doi: 10.1016/j.jmb.2023.168372. Epub 2023 Nov 17. J Mol Biol. 2024. PMID: 37979908 Review.
References
- Adamo A, Collis SJ, Adelman CA, Silva N, Horejsi Z, Ward JD, Martinez-Perez E, Boulton SJ, La Volpe A. Preventing nonhomologous end joining suppresses DNA repair defects of Fanconi anemia. Mol Cell. 2010;39:25–35. - PubMed
- Aiyar S, Sun JL, Li R. BRCA1: a locus-specific "liaison" in gene expression and genetic integrity. J Cell Biochem. 2005;94:1103–1111. - PubMed
- Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem. 2000;275:23899–23903. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1K99CA160574-01/CA/NCI NIH HHS/United States
- Z01 BC010283-10/ImNIH/Intramural NIH HHS/United States
- Z01 BC010283/ImNIH/Intramural NIH HHS/United States
- Y99 CA999999/CA/NCI NIH HHS/United States
- Z99 CA999999/ImNIH/Intramural NIH HHS/United States
- R01 DK043889/DK/NIDDK NIH HHS/United States
- K99 CA160574/CA/NCI NIH HHS/United States
- Z01 BC010959-01/ImNIH/Intramural NIH HHS/United States
- Z01 BC010959/ImNIH/Intramural NIH HHS/United States
- R37 HL052725/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous