Hedgehog signaling inhibition by the small molecule smoothened inhibitor GDC-0449 in the bone forming prostate cancer xenograft MDA PCa 118b - PubMed (original) (raw)
Hedgehog signaling inhibition by the small molecule smoothened inhibitor GDC-0449 in the bone forming prostate cancer xenograft MDA PCa 118b
Maria Karlou et al. Prostate. 2012 Nov.
Abstract
Background: Hedgehog signaling is a stromal-mesenchymal pathway central to the development and homeostasis of both the prostate and the bone. Aberrant Hedgehog signaling activation has been associated with prostate cancer aggressiveness. We hypothesize that Hedgehog pathway is a candidate therapeutic target in advanced prostate cancer. We confirm increased Hedgehog signaling in advanced and bone metastatic castrate resistant prostate cancer and examine the pharmacodynamic effect of Smoothened inhibition by the novel reagent GDC-0449 in an experimental prostate cancer model.
Methods: Hedgehog signaling component expression was assessed in tissue microarrays of high grade locally advanced and bone metastatic disease. Male SCID mice subcutaneously injected with the bone forming xenograft MDA PCa 118b were treated with GDC-0449. Hedgehog signaling in the tumor microenvironment was assessed by proteomic and species specific RNA expression and compared between GDC-0449 treated and untreated animals.
Results: We observe Hedgehog signaling in high grade locally advanced and bone marrow infiltrating disease. Evidence of paracrine activation of Hedgehog signaling in the tumor xenograft, was provided by increased Sonic Hedgehog expression in human tumor epithelial cells, coupled with increased Gli1 and Patched1 expression in the murine stromal compartment, while normal murine stroma did not exhibit Hh signaling expression. GDC-0449 treatment attenuated Hh signaling as evidenced by reduced expression of Gli1 and Ptch1. Reduction in proliferation (Ki67) was observed with no change in tumor volume.
Conclusions: GDC-0449 treatment is pharmacodynamically effective as evidenced by paracrine Hedgehog signaling inhibition and results in tumor cell proliferation reduction. Understanding these observations will inform the clinical development of therapy based on Hedgehog signaling inhibition.
Copyright © 2012 Wiley Periodicals, Inc.
Figures
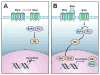
Figure 1. Schematic diagram of the Hh pathway
A: In the absence of ligand, the transmembrane receptor Patched (Ptch) normally inhibits the signaling function of another transmembrane protein, Smoothened (Smo), thereby blocking the expression of target genes. In that inactive situation, Suppressor of Fused (SuFu) prevents Gli from translocating to the nucleus. B: In the presence of the ligand Sonic Hedgehog (Shh), the repression of Smo is relieved after binding of Shh to Ptch. Smo further transduces a signal resulting in the release of transcription factor Gli from the cytoplasmic proteins Fused (Fu) and SuFu. In the active situation SuFu is inhibited by Fu and Gli is released, translocates to the nucleus and activates target genes transcription, such as Ptch and Gli1.
Figure 2. Expression of Shh pathway components in bone marrow metastatic (A,C,E,G) and primary (B,D,F,H) prostate cancer
Expression of Shh is high in both metastatic (A) and primary (B) prostate cancer. Ptch expression is higher in bone marrow metastasis (C) compared to primary prostate cancer (D). High expression of Gli2 and Gli1 is noted in metastatic and primary prostate cancer (E–H) (original magnification ×200).
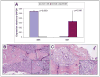
Figure 3. Upregulated epithelial Shh and stromal Gli1 mRNA expression and MDA PCa 118b tumor morphology
A. Mean Shh and Gli1 mRNA expression levels in cancer cells and in stromal compartment of MDA PCa 118b prostate cancer xenograft. B. Control and C. Treated tumors display similar histopathologic features and are characterized by robust metaplastic bone formation (H&E, original magnification ×100, insect: original magnification ×200).
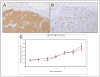
Figure 4. A. Gli2 and B. Ki67 expression, C. Mean tumor volume of MDA PCa 118b xenograft
A. High expression of Gli2 in a tumor treated with GDC-0449 (original magnification ×100). B. High expression of ki67 in the same tumor as A (original magnification ×100). C. Mean tumor volume of MDA PCa 118b prostate cancer xenograft during 21 days of treatment with small molecule Smo inhibitor GCD-0449. Control group with blue and GDC-0449 treated group with red color.
Similar articles
- TAK-441, a novel investigational smoothened antagonist, delays castration-resistant progression in prostate cancer by disrupting paracrine hedgehog signaling.
Ibuki N, Ghaffari M, Pandey M, Iu I, Fazli L, Kashiwagi M, Tojo H, Nakanishi O, Gleave ME, Cox ME. Ibuki N, et al. Int J Cancer. 2013 Oct 15;133(8):1955-66. doi: 10.1002/ijc.28193. Epub 2013 May 6. Int J Cancer. 2013. PMID: 23564295 - BMP4 promotes prostate tumor growth in bone through osteogenesis.
Lee YC, Cheng CJ, Bilen MA, Lu JF, Satcher RL, Yu-Lee LY, Gallick GE, Maity SN, Lin SH. Lee YC, et al. Cancer Res. 2011 Aug 1;71(15):5194-203. doi: 10.1158/0008-5472.CAN-10-4374. Epub 2011 Jun 13. Cancer Res. 2011. PMID: 21670081 Free PMC article. - Inhibition of hedgehog signaling improves the anti-carcinogenic effects of docetaxel in prostate cancer.
Mimeault M, Rachagani S, Muniyan S, Seshacharyulu P, Johansson SL, Datta K, Lin MF, Batra SK. Mimeault M, et al. Oncotarget. 2015 Feb 28;6(6):3887-903. doi: 10.18632/oncotarget.2932. Oncotarget. 2015. PMID: 25682877 Free PMC article. - Vismodegib.
Meiss F, Andrlová H, Zeiser R. Meiss F, et al. Recent Results Cancer Res. 2018;211:125-139. doi: 10.1007/978-3-319-91442-8_9. Recent Results Cancer Res. 2018. PMID: 30069764 Review. - Novel therapies for metastatic castrate-resistant prostate cancer.
Dayyani F, Gallick GE, Logothetis CJ, Corn PG. Dayyani F, et al. J Natl Cancer Inst. 2011 Nov 16;103(22):1665-75. doi: 10.1093/jnci/djr362. Epub 2011 Sep 13. J Natl Cancer Inst. 2011. PMID: 21917607 Free PMC article. Review.
Cited by
- Primary cilia are lost in preinvasive and invasive prostate cancer.
Hassounah NB, Nagle R, Saboda K, Roe DJ, Dalkin BL, McDermott KM. Hassounah NB, et al. PLoS One. 2013 Jul 2;8(7):e68521. doi: 10.1371/journal.pone.0068521. Print 2013. PLoS One. 2013. PMID: 23844214 Free PMC article. - Hedgehog Signaling and Truncated GLI1 in Cancer.
Doheny D, Manore SG, Wong GL, Lo HW. Doheny D, et al. Cells. 2020 Sep 17;9(9):2114. doi: 10.3390/cells9092114. Cells. 2020. PMID: 32957513 Free PMC article. Review. - Clinical Implications of Hedgehog Pathway Signaling in Prostate Cancer.
Suzman DL, Antonarakis ES. Suzman DL, et al. Cancers (Basel). 2015 Sep 29;7(4):1983-93. doi: 10.3390/cancers7040871. Cancers (Basel). 2015. PMID: 26426053 Free PMC article. Review. - The hedgehog inhibitor GANT61 sensitizes prostate cancer cells to ionizing radiation both in vitro and in vivo.
Gonnissen A, Isebaert S, McKee CM, Dok R, Haustermans K, Muschel RJ. Gonnissen A, et al. Oncotarget. 2016 Dec 20;7(51):84286-84298. doi: 10.18632/oncotarget.12483. Oncotarget. 2016. PMID: 27713179 Free PMC article. - Novel non-AR therapeutic targets in castrate resistant prostate cancer.
Toren PJ, Gleave ME. Toren PJ, et al. Transl Androl Urol. 2013 Sep;2(3):265-77. doi: 10.3978/j.issn.2223-4683.2013.09.09. Transl Androl Urol. 2013. PMID: 26816739 Free PMC article. Review.
References
- Efstathiou E, Troncoso P, Wen S, Do KA, Pettaway CA, Pisters LL, McDonnell TJ, Logothetis CJ. Initial modulation of the tumor microenvironment accounts for thalidomide activity in prostate cancer. Clin Cancer Res. 2007;13(4):1224–1231. - PubMed
- Cano P, Godoy A, Escamilla R, Dhir R, Onate SA. Stromal-epithelial cell interactions and androgen receptor-coregulator recruitment is altered in the tissue microenvironment of prostate cancer. Cancer Res. 2007;67(2):511–519. - PubMed
- Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22(18):2454–2472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical