Antidiabetic Effect of an Active Components Group from Ilex kudingcha and Its Chemical Composition - PubMed (original) (raw)
Antidiabetic Effect of an Active Components Group from Ilex kudingcha and Its Chemical Composition
Chengwu Song et al. Evid Based Complement Alternat Med. 2012.
Abstract
The leaves of Ilex kudingcha are used as an ethnomedicine in the treatment of symptoms related with diabetes mellitus and obesity throughout the centuries in China. The present study investigated the antidiabetic activities of an active components group (ACG) obtained from Ilex kudingcha in alloxan-induced type 2 diabetic mice. ACG significantly reduced the elevated levels of serum glycaemic and lipids in type 2 diabetic mice. 3-Hydroxy-3-methylglutaryl coenzyme A reductase and glucokinase were upregulated significantly, while fatty acid synthetase, glucose-6-phosphatase catalytic enzyme was downregulated in diabetic mice after treatment of ACG. These findings clearly provided evidences regarding the antidiabetic potentials of ACG from Ilex kudingcha. Using LC-DAD/HR-ESI-TOF-MS, six major components were identified in ACG. They are three dicaffeoylquinic acids that have been reported previously, and three new triterpenoid saponins, which were the first time to be identified in Ilex kudingcha. It is reasonable to assume that antidiabetic activity of Ilex kudingcha against hyperglycemia resulted from these six major components. Also, synergistic effects among their compounds may exist in the antidiabetic activity of Ilex kudingcha.
Figures
Figure 1
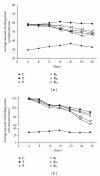
Food (a) and water (b) intake during treatment with ACG in type 2 diabetic mice. *P < 0.05 versus T group.
Figure 2
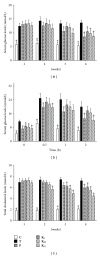
Serum glucose levels (a, b) and total cholesterol levels (c) during treatment with ACG in type 2 diabetic mice.
Figure 3
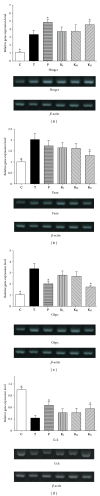
Gene expression analysis in the liver by real-time RT-PCR. (a) HMGCR; (b) FASN; (c) G6PC; (d) GCK. Significant differences were observed at P < 0.05* versus T groups. __β_-actin was used as a control to standardize the efficiency of each reaction. Gene expression was presented using a modification of the 2−ΔΔCt method [–14].
Figure 4
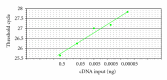
Fold-change on RT-PCR. The corresponding RT-PCR efficiencies were calculated according to the equation E = 10[−1/slope] [12].
Figure 5
LC-DAD chromatogram spectrum and HR-ESI-TOF-MSn negative mass spectrum of ACG.
Figure 6
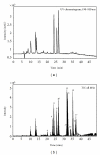
HR-ESI-TOF-MS/MS of three chlorogenic acids isomers identified in ACG and their structures. (a) Spectrum of Compound 1; (b) spectrum of Compound 2; (c) spectrum of Compound 3.
Figure 7
HR-ESI-TOF-MS/MS of three dicaffeoylquinic acid isomers identified in ACG. (a) Spectrum of Compound 8; (b) spectrum of Compound 9; (c) spectrum of Compound 10.
Figure 8
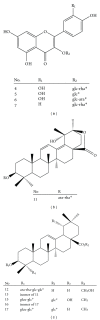
Structures of the flavonoids and triterpenoid saponins identified or characterized in ACG. *ara: arabinoside; rha: rhamnoside; glc: glucoside; glcu: glucuronide.
Similar articles
- Comparison of major phenolic constituents and in vitro antioxidant activity of diverse Kudingcha genotypes from Ilex kudingcha, Ilex cornuta, and Ligustrum robustum.
Zhu F, Cai YZ, Sun M, Ke J, Lu D, Corke H. Zhu F, et al. J Agric Food Chem. 2009 Jul 22;57(14):6082-9. doi: 10.1021/jf901020h. J Agric Food Chem. 2009. PMID: 19601659 - Simultaneous qualitative and quantitative evaluation of Ilex kudingcha C. J. tseng by using UPLC and UHPLC-qTOF-MS/MS.
Zhou J, Yi H, Zhao ZX, Shang XY, Zhu MJ, Kuang GJ, Zhu CC, Zhang L. Zhou J, et al. J Pharm Biomed Anal. 2018 Jun 5;155:15-26. doi: 10.1016/j.jpba.2018.02.037. Epub 2018 Feb 21. J Pharm Biomed Anal. 2018. PMID: 29605682 - Kudinoside-D, a triterpenoid saponin derived from Ilex kudingcha suppresses adipogenesis through modulation of the AMPK pathway in 3T3-L1 adipocytes.
Che Y, Wang Q, Xiao R, Zhang J, Zhang Y, Gu W, Rao G, Wang C, Kuang H. Che Y, et al. Fitoterapia. 2018 Mar;125:208-216. doi: 10.1016/j.fitote.2017.11.018. Epub 2017 Nov 21. Fitoterapia. 2018. PMID: 29170122 - Quantitative analysis of five kudinosides in the large-leaved Kudingcha and related species from the genus Ilex by UPLC-ELSD.
Li L, Peng Y, Ma G, He C, Feng Y, Lei Q, Xiao P. Li L, et al. Phytochem Anal. 2012 Nov-Dec;23(6):677-83. doi: 10.1002/pca.2372. Epub 2012 May 17. Phytochem Anal. 2012. PMID: 22593006 - The large-leaved Kudingcha (Ilex latifolia Thunb and Ilex kudingcha C.J. Tseng): a traditional Chinese tea with plentiful secondary metabolites and potential biological activities.
Li L, Xu LJ, Ma GZ, Dong YM, Peng Y, Xiao PG. Li L, et al. J Nat Med. 2013 Jul;67(3):425-37. doi: 10.1007/s11418-013-0758-z. Epub 2013 Mar 26. J Nat Med. 2013. PMID: 23529541 Free PMC article. Review.
Cited by
- Network pharmacology- and molecular docking-based analyses of the antihypertensive mechanism of Ilex kudingcha.
Liao F, Yousif M, Huang R, Qiao Y, Hu Y. Liao F, et al. Front Endocrinol (Lausanne). 2023 Aug 17;14:1216086. doi: 10.3389/fendo.2023.1216086. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37664830 Free PMC article. - Ursolic acid-enriched kudingcha extract enhances the antitumor activity of bacteria-mediated cancer immunotherapy.
Xu H, Piao L, Liu X, Jiang SN. Xu H, et al. BMC Complement Med Ther. 2022 May 4;22(1):123. doi: 10.1186/s12906-022-03612-2. BMC Complement Med Ther. 2022. PMID: 35509047 Free PMC article. - Therapeutic Properties of Edible Mushrooms and Herbal Teas in Gut Microbiota Modulation.
Vamanu E, Dinu LD, Pelinescu DR, Gatea F. Vamanu E, et al. Microorganisms. 2021 Jun 10;9(6):1262. doi: 10.3390/microorganisms9061262. Microorganisms. 2021. PMID: 34200833 Free PMC article. Review. - Chemical Composition, Bioactivity and Safety Aspects of Kuding Tea-From Beverage to Herbal Extract.
Wüpper S, Lüersen K, Rimbach G. Wüpper S, et al. Nutrients. 2020 Sep 12;12(9):2796. doi: 10.3390/nu12092796. Nutrients. 2020. PMID: 32932672 Free PMC article. Review. - Preventive effect of small-leaved Kuding tea (Ligustrum robustum) on high-diet-induced obesity in C57BL/6J mice.
Wu Y, Yang J, Liu X, Zhang Y, Lei A, Yi R, Tan F, Zhao X. Wu Y, et al. Food Sci Nutr. 2020 Jul 8;8(8):4512-4522. doi: 10.1002/fsn3.1758. eCollection 2020 Aug. Food Sci Nutr. 2020. PMID: 32884731 Free PMC article.
References
- Teng BS, Wang CD, Yang HJ, et al. A protein tyrosine phosphatase 1B activity inhibitor from the fruiting bodies of Ganoderma lucidum (Fr.) Karst and its hypoglycemic potency on streptozotocin-induced type 2 diabetic mice. Journal of Agricultural and Food Chemistry. 2011;59(12):6492–6500. - PubMed
- Shieh JP, Cheng KC, Chung HH, Kerh YF, Yeh CH, Cheng JT. Plasma glucose lowering mechanisms of catalpol, an active principle from roots of rehmannia glutinosa, in streptozotocin-induced diabetic rats. Journal of Agricultural and Food Chemistry. 2011;59(8):3747–3753. - PubMed
- Zhou ZW, Song CW, P M, et al. The hypoglycemic effect of hainan kuding tea on alloxan-induced diabetic mouse. Shi Zhen Guo Yi Guo Yao. 2011;15(1):22–24.
- Thuong PT, Su ND, Ngoc TM, et al. Antioxidant activity and principles of Vietnam bitter tea Ilex kudingcha. Food Chemistry. 2009;113(1):139–145.
- Huang X, Meng D, Rong Y. Determination of quertetin and kaempferol in the burgeon leaves and old leaves of Guangxi Kudingcha. The Chinese Journal of Modern Applied Pharmacy. 2005;5(2):383–385.
LinkOut - more resources
Full Text Sources