Redox signal-mediated sensitization of transient receptor potential melastatin 2 (TRPM2) to temperature affects macrophage functions - PubMed (original) (raw)
Redox signal-mediated sensitization of transient receptor potential melastatin 2 (TRPM2) to temperature affects macrophage functions
Makiko Kashio et al. Proc Natl Acad Sci U S A. 2012.
Abstract
The ability to sense temperature is essential for organism survival and efficient metabolism. Body temperatures profoundly affect many physiological functions, including immunity. Transient receptor potential melastatin 2 (TRPM2) is a thermosensitive, Ca(2+)-permeable cation channel expressed in a wide range of immunocytes. TRPM2 is activated by adenosine diphosphate ribose and hydrogen peroxide (H(2)O(2)), although the activation mechanism by H(2)O(2) is not well understood. Here we report a unique activation mechanism in which H(2)O(2) lowers the temperature threshold for TRPM2 activation, termed "sensitization," through Met oxidation and adenosine diphosphate ribose production. This sensitization is completely abolished by a single mutation at Met-214, indicating that the temperature threshold of TRPM2 activation is regulated by redox signals that enable channel activity at physiological body temperatures. Loss of TRPM2 attenuates zymosan-evoked macrophage functions, including cytokine release and fever-enhanced phagocytic activity. These findings suggest that redox signals sensitize TRPM2 downstream of NADPH oxidase activity and make TRPM2 active at physiological body temperature, leading to increased cytosolic Ca(2+) concentrations. Our results suggest that TRPM2 sensitization plays important roles in macrophage functions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
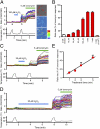
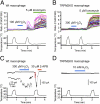
Heat-evoked responses of TRPM2 were elevated by H2O2 in a concentration- and time-dependent manner. (A) H2O2 (100 μM) enhanced heat-evoked increases in intracellular Ca2+ concentrations ([Ca2+]i) in DsRed(+) TRPM2-expressing cells (Left and Right Upper). Representative pseudocolor images of fluorescence intensity during heat stimulation before (a) and after (b) H2O2 treatment. (B) Each H2O2 concentration (10 μM, 30 μM, 60 μM, 100 μM, 1 mM, and 3 mM) was applied for 1 min as in A, and the heat-evoked [Ca2+]i increases after H2O2 treatment were normalized to the values in response to ionomycin for each experiment. Enhancement of the heat-evoked response was not observed in vector-transfected control cells (vector) or in TRPM2-expressing cells in the absence of extracellular Ca2+ (0 Ca2+), even at the highest H2O2 concentration (3 mM). Data are mean ± SEM (n = 5–13). (C and D) Representative traces of [Ca2+]i changes in TRPM2-expressing cells in response to heat before and after H2O2 (30 μM) treatment for 1 min (C) or 5 min (D) and later exposure to ionomycin (5 μM). (E) Heat-evoked responses of TRPM2 were elevated by prolonging H2O2 treatment. Data are mean ± SEM (n = 6 or 7). _R_2 = 0.97.
Fig. 2.
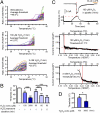
H2O2 reduced the temperature threshold for TRPM2 activation. (A) Representative traces of temperature-response profiles in heat stimulation without H2O2 (Top) or after H2O2 treatment at 100 μM (Middle) or 3 mM (Bottom) for 1 min. (B) Averaged data for heat stimulation without and after H2O2 treatment at 100 μM or 3 mM for 1 min and at 60 μM for 1, 3, and 5 min. Mean ± SEM (n = 5–8). ###P < 0.001 vs. H2O2-untreated; ***P < 0.001 between indicated pairs (ANOVA). (C) A heat-evoked current after 1 min exposure to pipette solution containing 100 μM H2O2 (Top) obtained by whole-cell recording. Representative Arrhenius plot traces are shown for temperature vs. density of heat-evoked currents with 100 μM H2O2 (Middle, using upper trace) or 3 mM (Bottom) after 1 min exposure. 2-APB, a TRPM2 inhibitor. (D) Averaged data for whole-cell recordings of heat-evoked currents after H2O2 treatment at 100 μM or 3 mM for 1 min. Data are mean ± SEM (n = 10 or 11). *P < 0.05 (t test).
Fig. 3.
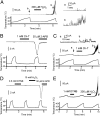
H2O2 sensitizes TRPM2 to heat in a membrane-delimited manner. (A) A heat-evoked current in a TRPM2-expressing cell at −60 mV in an inside-out configuration. Magnified traces (a and b) correspond to the currents shown by the arrows in the left trace. (B and C) Heat-evoked responses of TRPM2 were sensitized by chloramine-T, a membrane-permeant oxidant that preferentially oxidizes Met residues, in both whole-cell (B) and inside-out single-channel (C) recordings, where the magnified traces (a and b) correspond to the currents shown by the arrows in the above trace. (D and E) 5,5′-Dithiobis-2-nitrobenzoic acid, a membrane-impermeant Cys-specific oxidant, did not sensitize the heat response of TRPM2 in either whole-cell (D) or inside-out single-channel (E) recordings.
Fig. 4.
Structural basis for TRPM2 sensitization by H2O2. (A) Putative membrane topology of TRPM2, with the Met residues conserved between mouse and human TRPM2 indicated. Mutations to Ala were introduced at the Met residues indicated by red circles. (B–D) Reduction in the temperature threshold for TRPM2 activation by H2O2 treatment (100 μM for 1 or 3 min) was completely abolished in the M214A mutant. Shown are temperature-response profiles of heat-evoked [Ca2+]i increases observed in DsRed(+), WT (B), or M214A-expressing cells (C) and nonexpressing DsRed(−) cells (D). Data are mean ± SEM (n = 4 or 5). P < 0.05, Student t test or ANOVA, unless noted otherwise, between without and after treatment with H2O2. NS, not significant (ANOVA). (E) Sensitization of WT TRPM2 in the presence (+) or absence (−) of PJ-34. In the PJ(+) groups, the potent PARP inhibitor PJ-34 (1 μM) was present during the entire experiment. Data are mean ± SEM (n = 4–8). *P < 0.05; **P < 0.01; ***P < 0.001 vs. 0 min (ANOVA).
Fig. 5.
Sensitization is observed in WT macrophages, but not in TRPM2-deficient cells. (A and B) H2O2-induced sensitization of heat-evoked [Ca2+]i increases was observed in WT (A), but not in TRPM2-deficient (B) macrophages. (C and D) H2O2-induced sensitization of heat-evoked current was observed in WT (C), but not in TRPM2-deficient (D) macrophages. (C, Inset) A magnified trace corresponding to the red box shown in the upper trace.
Fig. 6.
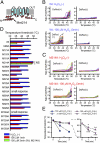
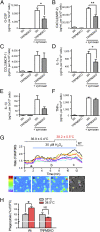
Zymosan-induced cytokine release and phagocytic activity in WT and TRPM2-deficient macrophages. (A–F) Amount of released cytokines from unstimulated and zymosan (50 μg/mL)-stimulated macrophages from WT and TRPM2-deficient mice. Data are mean ± SEM (n = 4–5). *P < 0.05; **_P_ < 0.01 (ANOVA). (_G_) A small temperature elevation caused further enhancement of H2O2 (30 μM)-induced [Ca2+]i increases in WT macrophages. Mean values for the normal and elevated temperatures are reported as mean ± SD. The lower panels show pseudocolor images of fluorescence intensity corresponding to the time points in the upper trace (a–c) and a phase-contrast image. Colored wedges in the phase-contrast image indicate the cells corresponding to each colored ratio trace. (_H_) Enhancement of phagocytic activity by elevated temperature (38.5 °C) was abolished in TRPM2-deficient macrophages. The ratio of cells that phagocytized zymosan particle(s) was normalized to the average values at 37 °C in each genotype. Data are mean ± SEM (_n_ = 3). *_P_ < 0.05; NS_P_ > 0.05 for 37 °C vs. 38.5 °C (Student t test).
Similar articles
- Protein kinase C-mediated phosphorylation of transient receptor potential melastatin type 2 Thr738 counteracts the effect of cytosolic Ca2+ and elevates the temperature threshold.
Kashio M, Masubuchi S, Tominaga M. Kashio M, et al. J Physiol. 2022 Oct;600(19):4287-4302. doi: 10.1113/JP283350. Epub 2022 Sep 12. J Physiol. 2022. PMID: 36042566 Free PMC article. - Sensitization of H2O2-induced TRPM2 activation and subsequent interleukin-8 (CXCL8) production by intracellular Fe(2+) in human monocytic U937 cells.
Shimizu S, Yonezawa R, Negoro T, Yamamoto S, Numata T, Ishii M, Mori Y, Toda T. Shimizu S, et al. Int J Biochem Cell Biol. 2015 Nov;68:119-27. doi: 10.1016/j.biocel.2015.09.005. Epub 2015 Sep 16. Int J Biochem Cell Biol. 2015. PMID: 26386353 - Role of H(2)O(2)-activated TRPM2 calcium channel in oxidant-induced endothelial injury.
Hecquet CM, Malik AB. Hecquet CM, et al. Thromb Haemost. 2009 Apr;101(4):619-25. Thromb Haemost. 2009. PMID: 19350103 Free PMC article. Review. - TRPM2: a multifunctional ion channel for calcium signalling.
Sumoza-Toledo A, Penner R. Sumoza-Toledo A, et al. J Physiol. 2011 Apr 1;589(Pt 7):1515-25. doi: 10.1113/jphysiol.2010.201855. Epub 2010 Dec 6. J Physiol. 2011. PMID: 21135052 Free PMC article. Review.
Cited by
- Involvement of TRPM2 in peripheral nerve injury-induced infiltration of peripheral immune cells into the spinal cord in mouse neuropathic pain model.
Isami K, Haraguchi K, So K, Asakura K, Shirakawa H, Mori Y, Nakagawa T, Kaneko S. Isami K, et al. PLoS One. 2013 Jul 30;8(7):e66410. doi: 10.1371/journal.pone.0066410. Print 2013. PLoS One. 2013. PMID: 23935822 Free PMC article. - Involvement of TRPM2 and TRPM8 in temperature-dependent masking behavior.
Ota W, Nakane Y, Kashio M, Suzuki Y, Nakamura K, Mori Y, Tominaga M, Yoshimura T. Ota W, et al. Sci Rep. 2019 Mar 6;9(1):3706. doi: 10.1038/s41598-019-40067-x. Sci Rep. 2019. PMID: 30842533 Free PMC article. - Does Cyclic ADP-Ribose (cADPR) Activate the Non-selective Cation Channel TRPM2?
Fliegert R, Riekehr WM, Guse AH. Fliegert R, et al. Front Immunol. 2020 Aug 11;11:2018. doi: 10.3389/fimmu.2020.02018. eCollection 2020. Front Immunol. 2020. PMID: 32903769 Free PMC article. Review. - Functional characterisation of a TRPM2 orthologue from the sea anemone Nematostella vectensis in human cells.
Kühn FJ, Kühn C, Lückhoff A. Kühn FJ, et al. Sci Rep. 2015 Jan 26;5:8032. doi: 10.1038/srep08032. Sci Rep. 2015. PMID: 25620041 Free PMC article. - Thermosensation and TRP Channels.
Tominaga M, Kashio M. Tominaga M, et al. Adv Exp Med Biol. 2024;1461:3-13. doi: 10.1007/978-981-97-4584-5_1. Adv Exp Med Biol. 2024. PMID: 39289270 Review.
References
- Kluger MJ, Kozak W, Conn CA, Leon LR, Soszynski D. Role of fever in disease. Ann N Y Acad Sci. 1998;856:224–233. - PubMed
- Hasday JD, Fairchild KD, Shanholtz C. The role of fever in the infected host. Microbes Infect. 2000;2:1891–1904. - PubMed
- Heiner I, Eisfeld J, Lückhoff A. Role and regulation of TRP channels in neutrophil granulocytes. Cell Calcium. 2003;33:533–540. - PubMed
- Inada H, Iida T, Tominaga M. Different expression patterns of TRP genes in murine B and T lymphocytes. Biochem Biophys Res Commun. 2006;350:762–767. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous