Nuclear p120 catenin unlocks mitotic block of contact-inhibited human corneal endothelial monolayers without disrupting adherent junctions - PubMed (original) (raw)
. 2012 Aug 1;125(Pt 15):3636-48.
doi: 10.1242/jcs.103267. Epub 2012 Apr 14.
Affiliations
- PMID: 22505615
- PMCID: PMC3445326
- DOI: 10.1242/jcs.103267
Nuclear p120 catenin unlocks mitotic block of contact-inhibited human corneal endothelial monolayers without disrupting adherent junctions
Ying-Ting Zhu et al. J Cell Sci. 2012.
Abstract
Contact inhibition ubiquitously exists in non-transformed cells that are in contact with neighboring cells. This phenomenon explains the poor regenerative capacity of in vivo human corneal endothelial cells during aging, injury and surgery. This study demonstrated that the conventional approach of expanding human corneal endothelial cells by disrupting contact inhibition with EDTA followed by bFGF activated canonical Wnt signaling and lost the normal phenotype to endothelial-mesenchymal transition, especially if TGFβ1 was added. By contrast, siRNA against p120 catenin (CTNND1) also uniquely promoted proliferation of the endothelial cells by activating trafficking of p120 catenin to the nucleus, thus relieving repression by nuclear Kaiso. This nuclear p120-catenin-Kaiso signaling is associated with activation of RhoA-ROCK signaling, destabilization of microtubules and inhibition of Hippo signaling, but not with activation of Wnt-β-catenin signaling. Consequently, proliferating human corneal endothelial cells maintained a hexagonal shape, with junctional expression of N-cadherin, ZO-1 and Na(+)/K(+)-ATPase. Further expansion of human corneal endothelial monolayers with a normal phenotype and a higher density was possible by prolonging treatment with p120 catenin siRNA followed by its withdrawal. This new strategy of perturbing contact inhibition by selective activation of p120-catenin-Kaiso signaling without disrupting adherent junction could be used to engineer surgical grafts containing normal human corneal endothelial cells to meet a global corneal shortage and for endothelial keratoplasties.
Figures
Fig. 1.
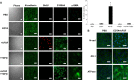
EMT with or without proliferation is caused by EDTA with or without bFGF and TGFβ1. (A) HCECs assume a fibroblastic shape after addition of bFGF and/or TGFβ. BrdU labeling is increased by 19% with EDTA-bFGF (n = 3, *P<0.05) but not with EDTA-TGFβ1 or EDTA-bFGF-TGFβ1. S100A4 staining was increased with EGTA-bFGF or TGFβ1, but became exclusively nuclear upon addition of EDTA-bFGF-TGFβ1. Cytoplasmic α-SMA staining was apparent with EDTA-TGFβ1 and was increased in EDTA-bFGF-TGFβ1-treated cells. In addition, N-cadherin switch from the membrane to the cytoplasm was apparent with EDTA-bFGF, EDTA-TGFβ1 or EDTA-bFGF-TGFβ1. (B) Membrane staining of N-cadherin, ZO-1 and Na+/K+-ATPase was disrupted in EDTA-bFGF-treated cells. Scale bars: 100 µm.
Fig. 2.
EMT induced by EDTA-bFGF is mediated by Wnt signaling. (A) Real-time PCR shows two- and threefold upregulation of β-catenin and LEF1 transcripts with EDTA-bFGF, respectively (n = 3, *P<0.05). (B) EDTA-bFGF promotes translocalization of β-catenin from the membrane to the nucleus and nuclear accumulation of LEF1. (C) Dot protein blotting confirmed that EDTA-bFGF causes a shift of β-catenin from the membrane to the nucleus and promotes nuclear accumulation of LEF1 (n = 3, *P<0.05; CN43, α-tubulin and histone were used as the loading control for membranous, cytosolic and nuclear compartments, respectively). (D) EDTA-bFGF activates Wnt signaling by activating the TCF/LEF promoter activity sixfold (n = 3, P<0.05). Addition of a specific Wnt inhibitor, XAV 939, completely abolishes the activity of TCF/LEF promoter and BrdU labeling. (E) EMT caused by addition of EDTA-bFGF was prevented by XAV 939. After addition of XAV 939, the staining of β-catenin, S100A4, N-cadherin, ZO-1 and Na+/K+-ATPase was similar to that from normal HCEC monolayers (cf. Fig. 1B, Fig. 2B). (F) Overexpression of S33Y β-catenin in HCEC monolayers treated with EGTA without growth factors increases BrdU labeling (by 21-fold, n = 3, P<0.05), nuclear β-catenin, LEF-1, S100A4 and cytoplasmic α-SMA, N-cadherin. (G) The activation of Wnt signaling by S33Y β-catenin was confirmed by Western blotting, indicating that nuclear β-catenin and LEF-1 are activated. Scale bars: 100 µm.
Fig. 3.
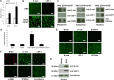
Mitotic block is uniquely unlocked by p120 siRNA without EMT. (A) HCEC monolayers treated with siRNA against p120 but not β-catenin, N-cadherin, or ZO-1, significantly promoted nuclear BrdU labeling up to 18-fold (n = 3, *P<0.05). (**B**) The proliferation promoted by p120 siRNA was not further promoted by additional bFGF (_n_ = 3, _P_>0.05). Addition of bFGF and/or TGFβ1 did not induce staining of S100A4 or α-SMA. However, addition of TGFβ1 abolished BrdU labeling promoted by p120 siRNA (n = 3, *P<0.05). (C) By contrast, the proliferation of HCEC monolayers decreased sevenfold (n = 3, P<0.05) upon addition of p120 siRNA when the cells were dissociated into single cells using EDTA-trypsin. Scale bars: 100 µm.
Fig. 4.
p120 siRNA triggers p120–Kaiso, but not Wnt signaling. (A) p120 siRNA downregulates expression of both p120 and Kaiso transcripts by 95% and 70%, respectively (n = 3, *P<0.05), but not β-catenin and LEF1 transcripts. (B) p120 siRNA induces nuclear translocalization of p120 (green), which colocalizes with nuclear BrdU labeling (red) (n = 3, *P<0.05) and decreases nuclear Kaiso staining, but does not affect membrane β-catenin or activate nuclear LEF1 staining. (C) Dot blotting confirmed that p120 siRNA increased the level of p120 and decreased that of Kaiso in the nuclear compartment, but did not alter that of β-catenin and LEF1. (D) In comparison, EDTA-bFGF did not alter the staining pattern of p120 and Kaiso. Scale bars: 100 µm. (E) Dot blotting confirmed that EDTA-bFGF did not affect p120 and Kaiso levels.
Fig. 5.
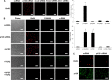
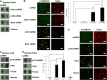
p120 nuclear translocation plays an important role in releasing nuclear Kaiso to unlock HCEC mitotic block. (A) The nuclear Kaiso level was significantly decreased by p120 siRNA and further by combined p120 and Kaiso siRNAs (n = 3, *P<0.05), but not by Kaiso siRNA (_n_ = 3, _P_>0.05). By contrast, the nuclear p120 level was not affected by Kaiso siRNA (not shown, n = 3, _P_>0.05). (B) Double immunostaining of p120 and BrdU (left) showed that BrdU labeling (red) correlated with nuclear p120 staining (green) was promoted by p120 siRNA but not Kaiso siRNA. A synergistic effect was noted by combined treatment with both p120 and Kaiso siRNAs (n = 3, *P<0.05). (C) Nuclear dot blotting shows the nuclear p120 protein level promoted by p120 siRNA is further enhanced by nocodazole, but is decreased by taxol (n = 3, *P<0.05). (D) Dot blotting shows the nuclear Kaiso protein level decreased by p120 siRNA is further decreased by nocodazole, but is increased by taxol to the control level (n = 3, *P<0.05). (E) The extent of nuclear p120 and BrdU labeling is negatively correlated with nuclear Kaiso levels, which is decreased by nocodazole but increased by taxol (n = 3, *P<0.05). Scale bars: 100 µm.
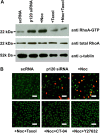
Fig. 6.
RhoA–ROCK signaling controls p120 nuclear translocation and its associated proliferation. (A) The level of active RhoA is promoted threefold by p120 siRNA and fourfold with addition of nocodazole. The level of active RhoA promoted by p120 siRNA or p120 siRNA+nocodazole is inhibited by taxol back to baseline levels. (B) Nuclear BrdU and p120 promoted by p120 siRNA+nocodazole was abolished by Taxol, CT-04 (RhoA inhibitor) or Y27632 (ROCK inhibitor). Scale bars: 100 µm.
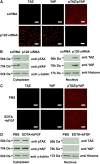
Fig. 7.
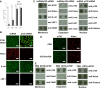
p120 knockdown but not EDTA-bFGF inhibits Hippo signaling. (A) Immunostaining shows that p120 siRNA causes nuclear accumulation of non-phosphorylated TAZ and YAP, and cytoplasmic deletion of phosphorylated TAZ and YAP when compared with addition of scRNA. (B) Because the same antibody detects both pYAP and pTAZ, Western blotting was used to confirm that both pTAZ and pYAP bands were decreased in the cytoplasm, whereas TAZ and YAP (detected by different antibodies) were increased in the nuclear extract. (C,D) By contrast, immunostaining (C) and western blotting (D) show that EDTA-bFGF fails to elicit such a change.
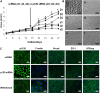
Fig. 8.
Prolonged treatment with p120 siRNA causes further expansion of HCEC monolayers without EMT. (A) The HCEC monolayer surface area reached a plateau when treated with scRNA (d1, d2 and d3; 1.7±0.4 mm2; n = 9, P<0.05), but was continuously promoted by p120 siRNA (D1, D2 and D3) (_P_<0.05 on day 18 except the D3/d3 pair, and on Day 21, 24 and 27 for all three pairs; 3.7±0.7 mm2; _n_ = 9, _P_<0.05) without cell enlargement in the center. (**B**) The HCEC density was 2241±104/mm2 when the Descemet membrane was stripped from the peripheral cornea (a). It increased to 2548±93/mm2 for HCEC monolayers cultured on Day 14 (b, _n_ = 5, _P_<0.05). For the control treated with scRNA, the HCEC density dropped to 2083±86/mm2 on Day 28 (c, _n_ = 5, _P_>0.05) and 1764±96 mm2 on Day 38 (d, n = 5, P<0.05), i.e. 10 days after withdrawal. By contrast, the HCEC density was maintained at 2316±79/mm2 on Day 28 (i.e. two weeks of p120 siRNA3 treatment) (e, _n_ = 5, _P_>0.05), and 2289±113/mm2 on Day 38 (i.e. 10 days after withdrawal, f, n = 5, _P_>0.05). (C) Prolonged p120 siRNA treatment results in nuclear translocation of p120 and dissolution of F-actin without disturbing the junctional staining pattern of N-cadherin, ZO-1 and Na+/K+-ATPase. Ten days after withdrawal of p120 siRNA, the staining pattern of p120 and F-actin reverted to the normal pattern whereas that of the other proteins remained unchanged. Scale bars: 100 µm.
Similar articles
- Knockdown of both p120 catenin and Kaiso promotes expansion of human corneal endothelial monolayers via RhoA-ROCK-noncanonical BMP-NFκB pathway.
Zhu YT, Han B, Li F, Chen SY, Tighe S, Zhang S, Tseng SC. Zhu YT, et al. Invest Ophthalmol Vis Sci. 2014 Mar 13;55(3):1509-18. doi: 10.1167/iovs.13-13633. Invest Ophthalmol Vis Sci. 2014. PMID: 24474278 Free PMC article. - Selective activation of p120ctn-Kaiso signaling to unlock contact inhibition of ARPE-19 cells without epithelial-mesenchymal transition.
Chen HC, Zhu YT, Chen SY, Tseng SC. Chen HC, et al. PLoS One. 2012;7(5):e36864. doi: 10.1371/journal.pone.0036864. Epub 2012 May 9. PLoS One. 2012. PMID: 22590627 Free PMC article. - LIF-JAK1-STAT3 signaling delays contact inhibition of human corneal endothelial cells.
Liu X, Tseng SC, Zhang MC, Chen SY, Tighe S, Lu WJ, Zhu YT. Liu X, et al. Cell Cycle. 2015;14(8):1197-206. doi: 10.1080/15384101.2015.1013667. Cell Cycle. 2015. PMID: 25695744 Free PMC article. - p120 catenin: an essential regulator of cadherin stability, adhesion-induced signaling, and cancer progression.
Kourtidis A, Ngok SP, Anastasiadis PZ. Kourtidis A, et al. Prog Mol Biol Transl Sci. 2013;116:409-32. doi: 10.1016/B978-0-12-394311-8.00018-2. Prog Mol Biol Transl Sci. 2013. PMID: 23481205 Free PMC article. Review. - Dancing in and out of the nucleus: p120(ctn) and the transcription factor Kaiso.
Daniel JM. Daniel JM. Biochim Biophys Acta. 2007 Jan;1773(1):59-68. doi: 10.1016/j.bbamcr.2006.08.052. Epub 2006 Sep 7. Biochim Biophys Acta. 2007. PMID: 17050009 Review.
Cited by
- EMT Transcription Factors Are Involved in the Altered Cell Adhesion under Simulated Microgravity Effect or Overloading by Regulation of E-cadherin.
Shi S, Li Q, Cao Q, Diao Y, Zhang Y, Yue L, Wei L. Shi S, et al. Int J Mol Sci. 2020 Feb 17;21(4):1349. doi: 10.3390/ijms21041349. Int J Mol Sci. 2020. PMID: 32079291 Free PMC article. - Phenotypic and functional characterization of corneal endothelial cells during in vitro expansion.
Frausto RF, Swamy VS, Peh GSL, Boere PM, Hanser EM, Chung DD, George BL, Morselli M, Kao L, Azimov R, Wu J, Pellegrini M, Kurtz I, Mehta JS, Aldave AJ. Frausto RF, et al. Sci Rep. 2020 May 4;10(1):7402. doi: 10.1038/s41598-020-64311-x. Sci Rep. 2020. PMID: 32366916 Free PMC article. - Manufacturing of human corneal endothelial grafts.
Zhu YT, Tighe S, Chen SL, Zhang Y, Chen SY, Kao WWY, Tseng SCG. Zhu YT, et al. Ocul Surf. 2023 Jul;29:301-310. doi: 10.1016/j.jtos.2023.05.004. Epub 2023 Jun 1. Ocul Surf. 2023. PMID: 37268293 Free PMC article. - Engineering of Human Corneal Endothelial Grafts.
Zhu YT, Tighe S, Chen SL, John T, Kao WY, Tseng SC. Zhu YT, et al. Curr Ophthalmol Rep. 2015 Sep;3(3):207-217. doi: 10.1007/s40135-015-0077-5. Epub 2015 Jun 27. Curr Ophthalmol Rep. 2015. PMID: 26509105 Free PMC article. - Transcription Factor 4 Regulates the Regeneration of Corneal Endothelial Cells.
Hwang JS, Yoon CK, Hyon JY, Chung TY, Shin YJ. Hwang JS, et al. Invest Ophthalmol Vis Sci. 2020 Apr 9;61(4):21. doi: 10.1167/iovs.61.4.21. Invest Ophthalmol Vis Sci. 2020. PMID: 32301972 Free PMC article. Retracted.
References
- Basile D. P., Friedrich J. L., Spahic J., Knipe N., Mang H., Leonard E. C., Changizi–Ashtiyani S., Bacallao R. L., Molitoris B. A., Sutton T. A. (2011). Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am. J. Physiol. Renal Physiol. 300, F721–F733 10.1152/ajprenal.00546.2010 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous