Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice - PubMed (original) (raw)
Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice
Kelvin C Luk et al. J Exp Med. 2012.
Abstract
The accumulation of misfolded proteins is a fundamental pathogenic process in neurodegenerative diseases. However, the factors that trigger aggregation of α-Synuclein (α-Syn), the principal component of the intraneuronal inclusions known as Lewy bodies (LBs), and Lewy neurites (LNs), which characterize Parkinson's disease (PD) and dementia with LBs (DLB), are poorly understood. We show here that in young asymptomatic α-Syn transgenic (Tg) mice, intracerebral injections of brain homogenates derived from older Tg mice exhibiting α-Syn pathology accelerate both the formation of intracellular LB/LN-like inclusions and the onset of neurological symptoms in recipient animals. Pathological α-Syn propagated along major central nervous system (CNS) pathways to regions far beyond injection sites and reduced survival with a highly reproducible interval from injection to death in inoculated animals. Importantly, inoculation with α-Syn amyloid fibrils assembled from recombinant human α-Syn induced identical consequences. Furthermore, we show for the first time that synthetic α-Syn fibrils are wholly sufficient to initiate PD-like LBs/LNs and to transmit disease in vivo. Thus, our data point to a prion-like cascade in synucleinopathies whereby cell-cell transmission and propagation of misfolded α-Syn underlie the CNS spread of LBs/LNs. These findings open up new avenues for understanding the progression of PD and for developing novel therapeutics.
Figures
Figure 1.
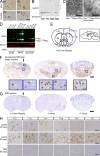
Transmission of α-Syn pathology after intracerebral inoculation of symptomatic aged M83 brain lysates harboring aggregated α-Syn. (A) Brainstem (BS) and spinal cord (SC) from symptomatic M83 mice used to prepare lysates for stereotaxic injections were stained with antibodies against pSyn (hyperphosphorylated α-Syn) or Syn303 (misfolded conformation of α-Syn) to detect α-Syn pathology. (B) Syn202 immunoblotting of lysates from symptomatic and asymptomatic M83 animals a sequential extraction with high-salt buffer (HS), HS + 1% Triton-X100 (TX), RIPA, and SDS buffer. Asterisks indicate high molecular weight α-Syn species. (C) Electron micrographs of α-Syn1-120Myc PFFs before and after sonication. (D) Immunoblot of α-Syn material used for intracerebral inoculation. The indicated amounts of symptomatic brain lysate (low-spin fraction) and recombinant α-Syn1-120Myc PFFs were separated and probed using either antibodies recognizing the N-terminal (SNL4) or C-terminal (Syn211) region of α-Syn, or against pSyn. Recombinant full-length human α-Syn monomer was used as standard. (E) Diagram illustrating route of stereotaxic injections. Pathological α-Syn was inoculated into the right hemisphere at subdural depths, indicated using a single needle tract. Exactly 2.5 µl of inoculum (either brain lysate or recombinant PFFs) was deposited into the striatum (Str) and cortex (Ctx). Inset shows sagittal view of injection sites. (F and G) Representative coronal brain sections from a young M83 mouse injected 90 d prior with symptomatic M83 brain lysate (F) or PBS (G). White arrows and arrowheads in insets show staining with anti-pSyn antibodies and LB/LN-like pathology. Bregma values denote the rostral/caudal level shown. Injection sites are indicated by black arrows. (H) pSyn pathology in young M83 mice injected with symptomatic aged M83 brain lysates 30 or 90 d prior, or with PBS 90 d prior. Injection sites are indicated by asterisks. Micrographs are representative of multiple lysate-injected M83 mice analyzed at 30 dpi (n = 3), 90 dpi (n = 12), or with PBS (n = 7). FrC (frontal cortex); Ctx (somatosensory cortex); Str (neostriatum); Thal (thalamus); BS (brainstem); SC (spinal cord). Bars: 50 µm (A and H); 500 nm (C); 1 mm (F and G); 25 µm (insets in F).
Figure 2.
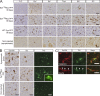
Recombinant α-Syn PFFs induce LBs and LNs in the CNS. (A) pSyn immunohistochemistry of various CNS regions in young M83 mice 30 or 90 d after intracerebral injection with either α-Syn1-120Myc or WT α-Syn PFFs. Asterisks indicate level of injection sites. Bottom panels show pSyn staining in aged symptomatic M83 mice. Immunohistochemical analyses were performed on multiple M83 mice after injection with PFFs assembled from α-Syn1-120Myc (30 d, n = 3; 90 d, n = 10) or from WT α-Syn (n = 4). (B) Brainstem sections from symptomatic lysate- or PFF-injected mice immunostained with antibodies to pathological α-Syn conformers (Syn506) or ubiquitin (Ubi). Thioflavin-S (ThS) staining was also performed to detect LB/LN-like pathology (inset, 60× magnification). (C) Colocalization between Syn506 and ThS in cytoplasmic and neuritic inclusions. Arrowheads denote a neurite positive for both markers. Bars: 50 µm (A); 25 µm (B); 5 µm (inset).
Figure 3.
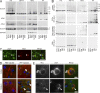
Biochemical analysis of α-Syn inoculated CNS tissue. (A) Immunoblots of brainstem samples sequentially extracted with the following: high-salt buffer (HS), HS+1% Triton-X (HS+Tx), 1% SDS (SDS) and formic acid (FA). Tissue was from untreated asymptomatic (Asym) M83 mice, aged symptomatic mice (Sym), or mice injected with PBS, symptomatic lysate, or α-Syn1-120Myc PFFs for 90 d. Immunoblots were probed with anti–α-Syn antibodies including anti–C terminus (Syn211), anti-phosphorylated Ser129 (pSyn), anti–mouse α-Syn (mSyn), or anti–α/β-Syn (Syn214). GAPDH is the loading control. Immunoblots are representative of two independent extraction experiments (n = 3 brains per group). (B) Various CNS regions from M83 mice injected with either symptomatic lysate or α-Syn1-120Myc PFFs for 90 d were sequentially extracted and probed using SNL4, which recognizes the N terminus of α-Syn. Samples from aged symptomatic M83 mice or mice injected with PBS are shown as positive controls. (C) Brainstem neurons of M83 mice injected with α-Syn1-120Myc PFFs (90 d) were double-immunostained using anti-pSyn and a rabbit polyclonal antibody specific for murine α-Syn (mSyn). Arrowheads show endogenous mSyn in pathological inclusions. (D) M83 were mice sacrificed 7 dpi with α-Syn1-120Myc PFFs. Tissue was double-immunostained for MAP-2 (red) and Myc (green) to detect internalization of exogenous Myc-tagged α-Syn by neurons. Cell nuclei were stained with DAPI (blue). Arrowheads denote intracellular Myc staining in cortical and striatal neurons. (E) Sections from α-Syn1-120Myc PFF-injected animals were double-immunolabeled against Myc and pSyn. Arrowheads denote accumulation of pSyn around internalized α-Syn. Bars: 50 µm (C); 15 µm (D); 10 µm (E).
Figure 4.
Intracerebral inoculation with pathological α-Syn reduces survival in M83 Tg mice. (A) Kaplan-Meier survival plots comparing lifespans of M83 mice injected (red) with either symptomatic brain lysate (n = 13) or α-Syn PFFs (n = 6). Uninjected M83 mice are shown in blue (n = 47). Gray bar indicates time of injection and age is shown on the horizontal axis. (P < 0.0001; χ2, 51.08; DF, 1). (B) Time until demise of young M83 mice after inoculation with pathological α-Syn (Sym, n = 19), asymptomatic M83 brain lysates (Asym), or PBS. Red arrowheads denote animals that received PFF injections. The demise of all mice inoculated with symptomatic M83 brain lysates or α-Syn PFFs occurred within 126 dpi (median 101 dpi), whereas mice injected with asymptomatic lysate- and PBS-treated animals (n = 4 each) remained disease free (P < 0.0001; χ2, 20.42; DF, 2).
Figure 5.
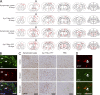
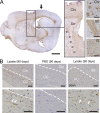
Distribution of α-Syn pathology after inoculation with pathological α-Syn. (A) Injections of symptomatic brain lysate or α-Syn1-120Myc PFFs were made to the right cortex and striatum (black arrows) of young healthy M83 mice. Maps denote the distribution of α-Syn LB- and LN-like pathology (red dots and lines, respectively) in coronal sections from injected mice sacrificed at either 30 or 90 dpi and immunostained with anti-pSyn. Representative plots are shown for mice injected with symptomatic lysate or α-Syn1-120Myc PFFs (n = 3–5 per group). (B) α-Syn pathology in dopaminergic neurons of inoculated M83 mice. Double-immunolabeling of tyrosine hydroxylase (TH, green) and pSyn (red) in the substantia nigra pars compacta of animals injected with α-Syn1-120Myc PFFs and sacrificed 90 d later. A subpopulation of dopaminergic neurons containing intracellular pSyn accumulations are indicated by arrows. (C) Glial fibrillary acidic protein (GFAP) immunostaining of the cortex, striatum, and brainstem of M83 mice injected with either symptomatic lysate, α-Syn1-120Myc PFFs, or PBS. Animals were sacrificed 90 dpi. (D) Double-immunostaining for pSyn and GFAP in the thalamus of M83 mouse 90 dpi with α-Syn1-120Myc PFFs. Arrows indicate astrocytes containing intracellular pSyn inclusions. Bars: 35 µm (B); 40 µm (C); 50 µm (D).
Figure 6.
Propagation and transmission of pathological α-Syn species in CNS. (A) Representative maps showing distribution of pSyn pathology 90 d after a single injection (black arrows) with symptomatic aged M83 brain lysate to the cortex or striatum in young M83 mice (n = 3 animals per group). (B) Sagittal brain section from a symptomatic lysate-injected M83 animal 90 dpi immunostained with anti-pSyn. Neocortex (Ctx), corpus callosum (CC), hypothalamus (Hypo), locus coeruleus (LC), cerebellar nuclei (CbN), and cerebellar white matter (CbW) are highlighted (insets of boxed areas, 40X magnification). (C) Possible routes of α-Syn propagation and transmission are illustrated. Gray denotes white matter tracts. (D) Diagram illustrating anatomical pattern of α-Syn accumulation in M83 mice 7, 30, or 90 dpi with either symptomatic brain lysate, α-Syn1-120Myc PFFs, or PBS. The distribution of α-Syn pathology in uninjected aged M83 animals is also shown. (E) Coronal section of axons in the corpus callosum (CC) and surrounding cortical (Ctx) and striatal (Str) tissue from a M83 mouse injected with symptomatic lysate. Immunostaining for pSyn (green) and microtubule-associated protein 2 (MAP2, red) were performed 90 dpi. The midline is also indicated (M). Bars: 1 mm (B); 50 µm (E).
Figure 7.
Pathological α-Syn species in major CNS pathways and white matter tracts. (A) Sagittal section of lateral forebrain from lysate-injected M83 animal showing LB/LN-like pathology labeled with anti-pSyn (inset) within cortex, striatum (Str) and internal capsule (IC; arrowheads in inset). Black arrow, injection site. (B) Anti-pSyn immunohistochemistry demonstrates α-Syn pathology (arrows) in corpus callosum and anterior commissure (AC) of lysate-injected animals, but not PBS-treated controls. Dashed lines demarcate white matter tracts. Bars: 1 mm (A); 50 µm (insets in A and B).
Similar articles
- Α-synuclein immunotherapy blocks uptake and templated propagation of misfolded α-synuclein and neurodegeneration.
Tran HT, Chung CH, Iba M, Zhang B, Trojanowski JQ, Luk KC, Lee VM. Tran HT, et al. Cell Rep. 2014 Jun 26;7(6):2054-65. doi: 10.1016/j.celrep.2014.05.033. Epub 2014 Jun 12. Cell Rep. 2014. PMID: 24931606 Free PMC article. - Potent prion-like behaviors of pathogenic α-synuclein and evaluation of inactivation methods.
Tarutani A, Arai T, Murayama S, Hisanaga SI, Hasegawa M. Tarutani A, et al. Acta Neuropathol Commun. 2018 Apr 18;6(1):29. doi: 10.1186/s40478-018-0532-2. Acta Neuropathol Commun. 2018. PMID: 29669601 Free PMC article. - Transmission of Soluble and Insoluble α-Synuclein to Mice.
Jones DR, Delenclos M, Baine AT, DeTure M, Murray ME, Dickson DW, McLean PJ. Jones DR, et al. J Neuropathol Exp Neurol. 2015 Dec;74(12):1158-69. doi: 10.1097/NEN.0000000000000262. J Neuropathol Exp Neurol. 2015. PMID: 26574670 Free PMC article. - Neuropathology of synuclein aggregates.
Duda JE, Lee VM, Trojanowski JQ. Duda JE, et al. J Neurosci Res. 2000 Jul 15;61(2):121-7. doi: 10.1002/1097-4547(20000715)61:2<121::AID-JNR1>3.0.CO;2-4. J Neurosci Res. 2000. PMID: 10878583 Review. - Formation and development of Lewy pathology: a critical update.
Jellinger KA. Jellinger KA. J Neurol. 2009 Aug;256 Suppl 3:270-9. doi: 10.1007/s00415-009-5243-y. J Neurol. 2009. PMID: 19711116 Review.
Cited by
- Development and validation of an expanded antibody toolset that captures alpha-synuclein pathological diversity in Lewy body diseases.
Altay MF, Kumar ST, Burtscher J, Jagannath S, Strand C, Miki Y, Parkkinen L, Holton JL, Lashuel HA. Altay MF, et al. NPJ Parkinsons Dis. 2023 Dec 7;9(1):161. doi: 10.1038/s41531-023-00604-y. NPJ Parkinsons Dis. 2023. PMID: 38062007 Free PMC article. - α-Synuclein-independent histopathological and motor deficits in mice lacking the endolysosomal Parkinsonism protein Atp13a2.
Kett LR, Stiller B, Bernath MM, Tasset I, Blesa J, Jackson-Lewis V, Chan RB, Zhou B, Di Paolo G, Przedborski S, Cuervo AM, Dauer WT. Kett LR, et al. J Neurosci. 2015 Apr 8;35(14):5724-42. doi: 10.1523/JNEUROSCI.0632-14.2015. J Neurosci. 2015. PMID: 25855184 Free PMC article. - A New Rise of Non-Human Primate Models of Synucleinopathies.
Teil M, Arotcarena ML, Dehay B. Teil M, et al. Biomedicines. 2021 Mar 9;9(3):272. doi: 10.3390/biomedicines9030272. Biomedicines. 2021. PMID: 33803341 Free PMC article. Review. - Corruption and spread of pathogenic proteins in neurodegenerative diseases.
Walker LC, LeVine H 3rd. Walker LC, et al. J Biol Chem. 2012 Sep 28;287(40):33109-15. doi: 10.1074/jbc.R112.399378. Epub 2012 Aug 9. J Biol Chem. 2012. PMID: 22879600 Free PMC article. Review. - Extracellular association of APP and tau fibrils induces intracellular aggregate formation of tau.
Takahashi M, Miyata H, Kametani F, Nonaka T, Akiyama H, Hisanaga S, Hasegawa M. Takahashi M, et al. Acta Neuropathol. 2015 Jun;129(6):895-907. doi: 10.1007/s00401-015-1415-2. Epub 2015 Apr 14. Acta Neuropathol. 2015. PMID: 25869641 Free PMC article.
References
- Abeliovich A., Schmitz Y., Fariñas I., Choi-Lundberg D., Ho W.H., Castillo P.E., Shinsky N., Verdugo J.M., Armanini M., Ryan A., et al. 2000. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 25:239–252 10.1016/S0896-6273(00)80886-7 - DOI - PubMed
- Beach T.G., Adler C.H., Sue L.I., Vedders L., Lue L., White Iii C.L., Akiyama H., Caviness J.N., Shill H.A., Sabbagh M.N., et al. ; Arizona Parkinson’s Disease Consortium 2010. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 119:689–702 10.1007/s00401-010-0664-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous