Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension - PubMed (original) (raw)
. 2012 May 25;110(11):1484-97.
doi: 10.1161/CIRCRESAHA.111.263848. Epub 2012 Apr 17.
Peter T Toth, John J Ryan, Zhigang Hong, Xichen Wu, Yong-Hu Fang, Thenappan Thenappan, Lin Piao, Hannah J Zhang, Jennifer Pogoriler, Yimei Chen, Erik Morrow, E Kenneth Weir, Jalees Rehman, Stephen L Archer
Affiliations
- PMID: 22511751
- PMCID: PMC3539779
- DOI: 10.1161/CIRCRESAHA.111.263848
Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension
Glenn Marsboom et al. Circ Res. 2012.
Abstract
Rationale: Pulmonary arterial hypertension (PAH) is a lethal syndrome characterized by pulmonary vascular obstruction caused, in part, by pulmonary artery smooth muscle cell (PASMC) hyperproliferation. Mitochondrial fragmentation and normoxic activation of hypoxia-inducible factor-1α (HIF-1α) have been observed in PAH PASMCs; however, their relationship and relevance to the development of PAH are unknown. Dynamin-related protein-1 (DRP1) is a GTPase that, when activated by kinases that phosphorylate serine 616, causes mitochondrial fission. It is, however, unknown whether mitochondrial fission is a prerequisite for proliferation.
Objective: We hypothesize that DRP1 activation is responsible for increased mitochondrial fission in PAH PASMCs and that DRP1 inhibition may slow proliferation and have therapeutic potential.
Methods and results: Experiments were conducted using human control and PAH lungs (n=5) and PASMCs in culture. Parallel experiments were performed in rat lung sections and PASMCs and in rodent PAH models induced by the HIF-1α activator, cobalt, chronic hypoxia, and monocrotaline. HIF-1α activation in human PAH leads to mitochondrial fission by cyclin B1/CDK1-dependent phosphorylation of DRP1 at serine 616. In normal PASMCs, HIF-1α activation by CoCl(2) or desferrioxamine causes DRP1-mediated fission. HIF-1α inhibition reduces DRP1 activation, prevents fission, and reduces PASMC proliferation. Both the DRP1 inhibitor Mdivi-1 and siDRP1 prevent mitotic fission and arrest PAH PASMCs at the G2/M interphase. Mdivi-1 is antiproliferative in human PAH PASMCs and in rodent models. Mdivi-1 improves exercise capacity, right ventricular function, and hemodynamics in experimental PAH.
Conclusions: DRP-1-mediated mitotic fission is a cell-cycle checkpoint that can be therapeutically targeted in hyperproliferative disorders such as PAH.
Figures
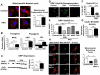
Figure 1. DRP1 activation causes excessive fission in human PAH PASMCs
A) Mitochondria are more fragmented in PAH versus control PASMCs. Quantification (mitochondrial fragmentation count) reveals a doubling of the number of individual mitochondria in PAH versus control PASMCs. Scale bar = 20μm. B) qRT-PCR reveals increased expression of the pro-fission proteins DRP1 and FIS1 and decreased expression of the pro-fusion protein MFN2 in human PAH PASMCs. C) Immunoblotting confirms the upregulation of DRP1 at the protein level (n=4 independent control cell lines and 5 independent PAH cell lines). Expression was normalized to actin for each sample. D) More PAH PASMCs are positive for DRP1 Ser616 phosphorylation than control PASMCs. Asterisks point at PASMCs with DRP1 phosphorylated at Ser616. Scale bar = 100μm. E) High levels of DRP1 Ser616 phosphorylation in PAH PASMCs versus control PASMCs. Only direct inhibition of CDK1 with 10μM RO-3306 and 16 hours of serum withdrawal (indicated by “–FBS” on the graph) decrease Ser616 phosphorylation levels. Inhibition of calmodulin with 5μM W7 or calcium/calmodulin kinase II with 20μM KN93 have no effect on DRP1 Ser616 phosphorylation. Approximately 100 PASMCs were counted for each group. F) Cyclin B1 expression was measured with flow cytometry and significantly higher in PAH PASMCs compared to control PASMCs. Serum withdrawal (“-FBS”) reduces cyclin B1 levels in PAH PASMCs. G) Activity of the cyclin B1/CDK1 complex was measured using a biosensor FRET probe. A higher yellow (YPet) over cyan (mCerulean) emission ratio indicates increased cyclin B1/CDK1 activity. PAH PASMCs have a higher activity compared to control PASMCs (n=10-11 cells/group). H) Photoactivation experiments confirm the decreased mitochondrial networking in PAH PASMCs. The mitochondrial networking factor (MNF) is lower in human PAH PASMCs and Mdivi-1 (25μM), an inhibitor of DRP1, reverses the fragmented phenotype and increases the MNF, demonstrating that increased DRP1 activity is the major determinant of the fragmented mitochondrial morphology in PAH. Scale bar = 10μm.
Figure 2. DRP1 inhibition reduces proliferation by causing G2/M arrest
A) PASMCs derived from PAH patients have more than double the rate of proliferation compared to control PASMCs, indicating that they maintain their hyperproliferative character in vitro. Mdivi-1 not only restores the mitochondrial network, but also dose-dependently reduces proliferation (n=5 independent cell lines). B) Mdivi-1 induced reduction in proliferation is due to G2/M cell cycle arrest. C-D) Knockdown of DRP1 with siRNA leads to a marked reduction in the proliferation rate of PAH PASMCs due to accumulation of PASMCs in the G2/M phase of the cell cycle. E) Upon activation, CDK1 is dephosphorylated at Thr14 and Tyr15. Mdivi-1 treatment (25μM) results in a clear activation of CDK1, suggesting that cells are either in late G2 phase or prometaphase of the cell cycle. Protein expression levels are expressed as arbitrary units (A.U.) versus untreated PASMCs after normalizing for actin expression. F) Prevention of microtubule assembly with 50ng/ml nocodazole synchronizes PAH PASMCs in the G2 phase of the cell cycle (increased percentage of cyclin B1 positive cells that have an increased DNA content). Removal of nocodazole allows cells to rapidly enter mitosis and after 4 hours the percentage of cyclin B1+ cells is normalized. However, if 25μM Mdivi-1 is added, cells are unable to progress through mitosis.
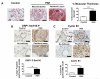
Figure 3. Activated DRP1 is present in remodeled pulmonary arteries of idiopathic PAH patients
A) In contrast to normal human lungs, idiopathic PAH patients have pulmonary blood vessels with excessive PASMC proliferation (“Muscularization”) and plexiform lesions which both contribute to an obstruction of the pulmonary blood vessels. Analysis of pulmonary blood vessels with an external diameter below 150μm demonstrates a significant thicker muscular coat. This together with the presence of plexiform lesions in all the analyzed patients confirms the diagnosis of PAH. Scale bar = 200μm. B) A significantly higher number of blood vessels stain positive for the active form of DRP1 (characterized by Ser616 phosphorylation) in human PAH versus control lungs. Approximately 150 blood vessels were analyzed in each group. Scale bar = 50μm. C) Cyclin B1 together with Cyclin-dependent kinase 1 leads to phosphorylation of DRP1 on Ser616. Cyclin B1 staining was present in nonvascular tissues in control lungs, but was almost absent in the PASMC nuclei of small pulmonary arteries of control lungs. In contrast, there was clear nuclear accumulation (activation) of cyclin B1 in human PAH blood vessels. Approximately 120 PASMC nuclei were analyzed in each group. Scale bar = 50μm.
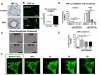
Figure 4. HIF-1α is activated in lungs and PASMCs from PAH patients which is necessary for proliferation and leads to mitochondrial fragmentation
A) Immunohistochemistry for HIF-1α reveals that PAH patients have pulmonary blood vessels that stain positive in the media for HIF-1α. Scale bar = 40μm. B) Cultured PASMCs from idiopathic PAH patients maintain elevated HIF-1α expression levels in vitro despite being cultured at ambient oxygen concentrations. Approximately 100 PASMCs were analyzed for each group. Scale bar = 100μm. C) Dose response of chetomin leads to only small changes in cellular morphology in human PASMCs. Scale bar = 200μm. D) Inhibition of HIF-1α signaling with chetomin leads to a dose-dependent reduction in proliferation in both control and PAH PASMCs. E) Chetomin dose-dependently reduces DRP1 Ser616 phosphorylation in human PASMCs. Approximately 250 nuclei were counted for each group. F) Activating HIF-1α with CoCl2 (500μM) leads to a rapid mitochondrial fragmentation, reminiscent of the fragmented morphology observed in PAH PASMCs.
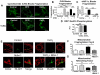
Figure 5. Fragmentation of mitochondria by cobalt is dependent on HIF-1α and DRP1 activation
A-B) Inhibition of protein translation with 50μg/ml cycloheximide (CHX) prevents mitochondrial fragmentation, as measured by the mitochondrial fragmentation count, induced by either 500μM CoCl2 or 100μM desferrioxamine (DFO). Scale bar = 20μm. C) In rat PASMCs treated with scrambled siRNA, CoCl2 induces mitochondrial fragmentation. In contrast, when PASMCs were transfected with siRNA against HIF-1α the fragmentation is largely prevented (n=6 cells/group). D) PASMCs were treated with 500μM CoCl2 for either 2 or 24 hours. Immunoblotting demonstrates that the ratio of phosphorylated DRP1 Ser616 was increased at both time points. Protein expression levels are normalized to total DRP1 signal (arbitrary units (A.U.)). E-F) We cotransfected PASMCs with mitochondrial matrix-targeted DsRed and photoactivatable GFP. Mdivi-1 (25μM) restores the mitochondrial network (lowers the mitochondrial fragmentation count) (E) and increases the mitochondrial networking factor (F). Scale bar = 10μm.
Figure 6. Inhibition of DRP1 using Mdivi-1 prevents cobalt-induced PAH in vivo
A-F) Chronic treatment with CoCl2 for 4-weeks causes pulmonary hypertension which is prevented by Mdivi-1. Hematocrit is increased by CoCl2 treatment (A). Exercise capacity (the distance rats can run until exhaustion on a treadmill) is reduced in cobalt-treated rats and Mdivi-1 treatment normalizes exercise capacity (B). Cobalt decreases pulmonary artery acceleration time (PAAT), indicative of increased pulmonary arterial pressures. Mdivi-1 partially normalizes the PAAT (C). CoCl2 doubles the PVR (defined as: mean pulmonary artery pressure – left ventricular end diastolic pressure divided by the cardiac output) and Mdivi-1 significantly reduces PVR (D). Right ventricular systolic pressure is increased by CoCl2 treatment (E), but this is not induced by left ventricular dysfunction as the left ventricular end diastolic pressure (LVEDP) is not changed by any treatment (F). Post mortem determination of right ventricular fractional weight (RV/LV+S) showed that CoCl2 increased right ventricular hypertrophy which is normalized by Mdivi-1 treatment (G). Immunohistochemistry for von Willebrand factor (green) and smooth muscle cell actin (Red) reveals that small precapillary resistance vessels in the lungs of CoCl2-treated animals have a thicker media versus control lungs and Mdivi-1 partially reverses this (H-I). Scale bar = 50μm. J) Electron microscopy imaging of pulmonary arteries derived from control animals and rats treated with CoCl2 and CoCl2 + Mdivi-1. Pulmonary artery smooth muscle cells were identified at a low magnification (5900x) by their characteristic cytoplasmic phenotype, their close proximity to collagen fibers and the absence of Weibel Palade bodies (upper panels, scale bar = 1μm). Higher resolution images (25000x, lower panels, scale bar = 200nm) were then acquired to measure mitochondrial size. Note the smaller mitochondrial size in the CoCl2 rats and the normalization in the CoCl2 + Mdivi-1 rats.
Figure 7. Therapeutic benefit of Mdivi-1 in the chronic hypoxia model
A) Chronic hypoxia (4 weeks, 10% oxygen) leads to a significant decrease in treadmill exercise time compared to controls, which is not observed when rats are treated with Mdivi-1. At the same time, pulmonary artery acceleration time (PAAT), tricuspid annular plane systolic excursion (TAPSE) and right ventricular fractional weight were significantly improved by Mdivi-1 treatment, n=10 animals/group. B-C) Lung sections of rats exposed to chronic hypoxia were stained with smooth muscle cell actin (green). The degree of pulmonary artery muscularization is significantly decreased in chronic hypoxia animals treated with Mdivi-1 compared to DMSO treated animals. Scale bar = 50μm. D) The percentage of proliferating PASMCs is significantly reduced after Mdivi-1 treatment.
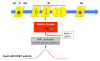
Figure 8. Schematic representation of how the cell and mitochondrial cycles interact
Activation of the mitochondrial GTPase DRP1 reflects regulatory kinases (cyclin B1/CDK1) that respond to signals from growth factors. In pulmonary hypertensive patients, cyclin B1 levels are elevated leading to activation of DRP1. By inhibiting fission (which is required for mitochondrial division) the cell cycle is halted causing cells to be locked in G2/M arrest. The intersection of the mitochondrial and cell cycles is cyclin B1/CDK1-mediated mitotic fission and this exposes an Achilles heel for rapidly proliferating cells, that can be therapeutically targeted.
Comment in
Similar articles
- Epigenetic Dysregulation of the Dynamin-Related Protein 1 Binding Partners MiD49 and MiD51 Increases Mitotic Mitochondrial Fission and Promotes Pulmonary Arterial Hypertension: Mechanistic and Therapeutic Implications.
Chen KH, Dasgupta A, Lin J, Potus F, Bonnet S, Iremonger J, Fu J, Mewburn J, Wu D, Dunham-Snary K, Theilmann AL, Jing ZC, Hindmarch C, Ormiston ML, Lawrie A, Archer SL. Chen KH, et al. Circulation. 2018 Jul 17;138(3):287-304. doi: 10.1161/CIRCULATIONAHA.117.031258. Epub 2018 Feb 5. Circulation. 2018. PMID: 29431643 Free PMC article. - Hypoxia induces pulmonary artery smooth muscle dysfunction through mitochondrial fragmentation-mediated endoplasmic reticulum stress.
Zhuan B, Wang X, Wang MD, Li ZC, Yuan Q, Xie J, Yang Z. Zhuan B, et al. Aging (Albany NY). 2020 Nov 18;12(23):23684-23697. doi: 10.18632/aging.103892. Epub 2020 Nov 18. Aging (Albany NY). 2020. PMID: 33221740 Free PMC article. - Novel Drp1 GTPase Inhibitor, Drpitor1a: Efficacy in Pulmonary Hypertension.
Wu D, Jansen-van Vuuren RD, Dasgupta A, Al-Qazazi R, Chen KH, Martin AY, Mewburn JD, Alizadeh E, Lima PDA, Jones O, Colpman P, Bentley RET, VandenBroek MM, Breault NM, Emon IM, Yerramilli VS, Jedlovčnik L, Zhao YY, Wells M, Sutendra G, Ormiston ML, Archer SL. Wu D, et al. Hypertension. 2024 Oct;81(10):2189-2201. doi: 10.1161/HYPERTENSIONAHA.124.22822. Epub 2024 Aug 20. Hypertension. 2024. PMID: 39162036 - Mitochondrial dynamics in pulmonary arterial hypertension.
Ryan J, Dasgupta A, Huston J, Chen KH, Archer SL. Ryan J, et al. J Mol Med (Berl). 2015 Mar;93(3):229-42. doi: 10.1007/s00109-015-1263-5. Epub 2015 Feb 13. J Mol Med (Berl). 2015. PMID: 25672499 Free PMC article. Review. - Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1.
Chang CR, Blackstone C. Chang CR, et al. Ann N Y Acad Sci. 2010 Jul;1201:34-9. doi: 10.1111/j.1749-6632.2010.05629.x. Ann N Y Acad Sci. 2010. PMID: 20649536 Free PMC article. Review.
Cited by
- Mitochondrial fission - a drug target for cytoprotection or cytodestruction?
Rosdah AA, K Holien J, Delbridge LM, Dusting GJ, Lim SY. Rosdah AA, et al. Pharmacol Res Perspect. 2016 Apr 21;4(3):e00235. doi: 10.1002/prp2.235. eCollection 2016 Jun. Pharmacol Res Perspect. 2016. PMID: 27433345 Free PMC article. Review. - Mitochondrial remodeling: Rearranging, recycling, and reprogramming.
Gottlieb RA, Bernstein D. Gottlieb RA, et al. Cell Calcium. 2016 Aug;60(2):88-101. doi: 10.1016/j.ceca.2016.04.006. Epub 2016 Apr 20. Cell Calcium. 2016. PMID: 27130902 Free PMC article. Review. - Notoginsenoside R1 improves intestinal microvascular functioning in sepsis by targeting Drp1-mediated mitochondrial quality imbalance.
Hou D, Liu R, Hao S, Dou Y, Chen G, Liu L, Li T, Cao Y, Huang H, Duan C. Hou D, et al. Pharm Biol. 2024 Dec;62(1):250-260. doi: 10.1080/13880209.2024.2318349. Epub 2024 Feb 22. Pharm Biol. 2024. PMID: 38389274 Free PMC article. - Detection of a Mitochondrial Fragmentation and Integrated Stress Response Using the Cell Painting Assay.
Rezaei Adariani S, Agne D, Koska S, Burhop A, Seitz C, Warmers J, Janning P, Metz M, Pahl A, Sievers S, Waldmann H, Ziegler S. Rezaei Adariani S, et al. J Med Chem. 2024 Aug 8;67(15):13252-13270. doi: 10.1021/acs.jmedchem.4c01183. Epub 2024 Jul 17. J Med Chem. 2024. PMID: 39018123 Free PMC article. - Novel super-resolution capable mitochondrial probe, MitoRed AIE, enables assessment of real-time molecular mitochondrial dynamics.
Lo CY, Chen S, Creed SJ, Kang M, Zhao N, Tang BZ, Elgass KD. Lo CY, et al. Sci Rep. 2016 Aug 5;6:30855. doi: 10.1038/srep30855. Sci Rep. 2016. PMID: 27492961 Free PMC article.
References
- Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, 3rd, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in bmpr2, encoding a tgf-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84. - PubMed
- Thomson JR, Machado RD, Pauciulo MW, Morgan NV, Humbert M, Elliott GC, Ward K, Yacoub M, Mikhail G, Rogers P, Newman J, Wheeler L, Higenbottam T, Gibbs JS, Egan J, Crozier A, Peacock A, Allcock R, Corris P, Loyd JE, Trembath RC, Nichols WC. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding bmpr-ii, a receptor member of the tgf-beta family. J Med Genet. 2000;37:741–745. - PMC - PubMed
- Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thebaud B, Husain AN, Cipriani N, Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: A basis for excessive cell proliferation and a new therapeutic target. Circulation. 2010;121:2661–2671. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1RC1HL099462-01/HL/NHLBI NIH HHS/United States
- R01-HL071115/HL/NHLBI NIH HHS/United States
- RC1 HL099462/HL/NHLBI NIH HHS/United States
- R01 HL071115/HL/NHLBI NIH HHS/United States
- R01 GM094220/GM/NIGMS NIH HHS/United States
- R01-GM094220/GM/NIGMS NIH HHS/United States
- R01 HL113003/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous