Phosphoregulation of Spc105 by Mps1 and PP1 regulates Bub1 localization to kinetochores - PubMed (original) (raw)
Phosphoregulation of Spc105 by Mps1 and PP1 regulates Bub1 localization to kinetochores
Nitobe London et al. Curr Biol. 2012.
Abstract
Kinetochores are the macromolecular complexes that interact with microtubules to mediate chromosome segregation. Accurate segregation requires that kinetochores make bioriented attachments to microtubules from opposite poles. Attachments between kinetochores and microtubules are monitored by the spindle checkpoint, a surveillance system that prevents anaphase until every pair of chromosomes makes proper bioriented attachments. Checkpoint activity is correlated with the recruitment of checkpoint proteins to the kinetochore. Mps1 is a conserved protein kinase that regulates segregation and the spindle checkpoint, but few of the targets that mediate its functions have been identified. Here, we show that Mps1 is the major kinase activity that copurifies with budding yeast kinetochore particles and identify the conserved Spc105/KNL-1/blinkin kinetochore protein as a substrate. Phosphorylation of conserved MELT motifs within Spc105 recruits the Bub1 protein to kinetochores, and this is reversed by protein phosphatase I (PP1). Spc105 mutants lacking Mps1 phosphorylation sites are defective in the spindle checkpoint and exhibit growth defects. Together, these data identify Spc105 as a key target of the Mps1 kinase and show that the opposing activities of Mps1 and PP1 regulate the kinetochore localization of the Bub1 protein.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
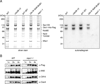
Figure 1. Mps1 kinase activity specifically co-purifies with native kinetochore complexes
(A) WT (SBY8253), cdc28-13 (SBY8716), ipl1-321 (SBY8712) Ubi-R-TetR-BUB1 (SBY8920) and mps1-1 (SBY8726) strains were shifted to conditions to inactivate the kinases and kinetochores were purified by α-Flag immunoprecipitation of Dsn1-His-Flag. The kinetochores were treated with γ-32P-ATP and eluted with 3×-Flag peptide. Particle composition was analyzed by silver-stained SDS-PAGE (left) and radioactive incorporation was analyzed by autoradiography (right). (B) Wild type and mps1-1 kinetochore complexes prepared as in (A) were analyzed by immunoblotting with the indicated antibodies. The relative amount of sample loaded in each lane is indicated at top.
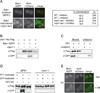
Figure 2. The association of Bub1 with kinetochores is regulated by Mps1-mediated phosphorylation
(A) pMet-Cdc20 BUB1-3GFP MTW1-mCherry cells containing MPS1 (SBY10318) or mps1-as1 (SBY10282) were arrested in mitosis by Cdc20 depletion and treated with nocodazole. They were then incubated with (+ inhibitor) or without (− inhibitor) 1NM-PP1 and analyzed for co-localization between Bub1 and Mtw1. Scale bar = 5.0 microns. (B) Kinetochore particles from strains containing Bub1-3GFP and MPS1 (SBY8502) or mps1-1 (SBY9348) were purified and analyzed by immunoblotting. (C) Kinetochore particles were purified from a strain containing Bub1-3GFP (SBY8502). The particles were treated with PP1 in the presence or absence of phosphatase inhibitors and the bound and unbound pools were analyzed by immunoblotting. (D) Kinetochore particles were purified from strains containing Bub1-3GFP and MPS1 (SBY8502) or mps1-1 (SBY9348). The particles were treated with PP1 and then incubated with +/− ATP. They were then incubated in a lysate from a WT strain (SBY8502) and analyzed by immunoblotting. (E) BUB1-3GFP NUF2-mCherry pGAL-MPS1 cells (SBY10271) were arrested in G1 and then treated with or without galactose. The co-localization of Bub1 with Nuf2 was analyzed by microscopy. Scale bar = 5.0 microns.
Figure 3. Mps1 and Glc7 regulate the Spc105/Bub1 interaction
(A) Kinetochore particles from WT (SBY8253), SPC105-3GFP (SBY8750), mps1-1 (SBY8726), and SPC105-3GFP mps1-1 (SBY9099) strains were treated as in Fig 1A, as was a control purification from untagged strain SBY3. Recombinant GST-Mps1 was incubated with the mps1-1 samples. (B) Spc105-3Flag was immunoprecipitated from BUB1-13myc control cells (SBY3269) grown at 23 °C and from SPC105-3Flag (SBY7420), SPC105-3Flag BUB1-13myc (SBY9226) and SPC105-3Flag BUB1-13myc mps1-1 (SBY9476) cells shifted to 37 °C for 2 hours before harvest. Samples were analyzed by immunoblotting. (C) Spc105-3Flag was immunoprecipitated from WT (SBY9226), pGAL-MPS1 (SBY10100), and pGAL-GLC7 (SBY9971) cells containing BUB1-13myc and analyzed by immunoblotting. Control strains containing BUB1-13myc but lacking Spc105-3Flag are SBY4020 (pGAL-GLC7), SBY3269 (WT) and SBY4587 (pGAL-Mps1). (D) pMET-CDC20 cells containing Spc105-Flag and/or Bub1-13myc (SBY10464, SBY10364, SBY10465 and SBY10466) were arrested in metaphase and then galactose was added to induce Mps1 overexpression and cells were processed as in (C). (E) Spc105-3Flag was purified from cells arrested in G1 and treated with galactose for one hour to induce Mps1 overexpression. The strains used were BUB1-13myc (SBY3269), BUB1-13myc pGAL-MPS1 (SBY4587), SPC105-3Flag (SBY7492), SPC105-3Flag pGal-MPS1 (SBY9952), SPC105-3Flag BUB1-13myc (SBY9226), SPC105-3FLAG BUB1-13myc pGAL-MPS1 (SBY10100). (F) GLC7 (SBY9337) and pGAL-GLC7 (SBY10479) cells containing BUB1-3GFP and MTW1-mCherry were induced with or without galactose for 1 hour. The percentage of cells with Bub1-3GFP foci localizing to the kinetochore was quantified by microscopy. Scale bar = 5.0 microns.
Figure 4. The conserved MELT motifs in Spc105 regulate Bub1 localization to kinetochores and the spindle checkpoint
(A) A diagram of the N-terminal region of Spc105 shows the location of 6 MELT-like motifs illustrated as green bars. Residues that were detected as phosphorylated after Mps1 treatment are indicated with an asterisk. The N-terminus of Spc105 is denoted by “N” and the middle domain of the protein by “M”. Table shows MELT-like motifs (bolded) and surrounding sequence. (B) Kinetochores were purified (silver stain, right) from strains containing SPC105-9myc (SBY10267) or spc105-6A-9myc (SBY10265). They were incubated with radioactive ATP and the corresponding phosphate incorporation was quantified (autorad, left). Standard deviation of normalized Spc105 signal is +/− 5% (n=3). (C) Bub1-GFP was immunoprecipitated from strains containing Spc105-9myc (SBY10266) or Spc105-6A-9myc (SBY10264). (D) Dsn1-Flag was immunoprecipitated from strains containing SPC105-9myc Bub1-3GFP (SBY10426) or Spc105-6A-9myc Bub1-3GFP (SBY10422). (E) Kinetochore particles were purified from strains containing Bub1-3GFP and SPC105 (SBY10426) or spc105-6A (SBY10422). The particles were treated with PP1 and then incubated with +/− ATP. They were then incubated in lysates from WT strains (SBY10426) containing Bub1-3GFP and analyzed by immunoblotting. (F) Strains containing BUB1-GFP MTW1-mCherry and SPC105-9myc (SBY10266) or spc105-6A-9myc (SBY10264) were arrested in nocodazole for 60 minutes. The percentage of cells showing co-localization of Bub1 with Mtw1 was quantified. Note that Bub1 is enriched on kinetochores that have detached from microtubules and are no longer clustered together [18]. (G) Five-fold serial dilutions of WT (SBY3), SPC105-9myc (SBY10267), _mad2_Δ(SBY292), Ubi-R-TetR-BUB1 (SBY8920), or two independently generated spc105-6A-9myc strains (SBY10265, SBY10254) were analyzed for growth on YPD with doxycycline in the presence or absence of 10 µg/ml benomyl. (H) Pds1 levels were analyzed in SPC105-6His (SBY10373) or spc105-6A-6His (SBY10374) cells released from G1 into nocodazole. Pgk1 is a loading control. (H) Model for silencing of the spindle checkpoint. When kinetochores are not correctly attached to microtubules, Mps1 phosphorylates Spc105 to recruit Bub1/3. Proper microtubule attachment promotes PP1 interaction with Spc105, leading to dephosphorylation of Spc105 and release of Bub1/3 to promote checkpoint silencing.
Similar articles
- Dual mechanisms regulate the recruitment of spindle assembly checkpoint proteins to the budding yeast kinetochore.
Aravamudhan P, Chen R, Roy B, Sim J, Joglekar AP. Aravamudhan P, et al. Mol Biol Cell. 2016 Nov 7;27(22):3405-3417. doi: 10.1091/mbc.E16-01-0007. Epub 2016 May 11. Mol Biol Cell. 2016. PMID: 27170178 Free PMC article. - KNL1/Spc105 recruits PP1 to silence the spindle assembly checkpoint.
Rosenberg JS, Cross FR, Funabiki H. Rosenberg JS, et al. Curr Biol. 2011 Jun 7;21(11):942-7. doi: 10.1016/j.cub.2011.04.011. Curr Biol. 2011. PMID: 21640906 Free PMC article. - Delineating the contribution of Spc105-bound PP1 to spindle checkpoint silencing and kinetochore microtubule attachment regulation.
Roy B, Verma V, Sim J, Fontan A, Joglekar AP. Roy B, et al. J Cell Biol. 2019 Dec 2;218(12):3926-3942. doi: 10.1083/jcb.201810172. Epub 2019 Oct 24. J Cell Biol. 2019. PMID: 31649151 Free PMC article. - Spindle checkpoint silencing: PP1 tips the balance.
Lesage B, Qian J, Bollen M. Lesage B, et al. Curr Biol. 2011 Nov 8;21(21):R898-903. doi: 10.1016/j.cub.2011.08.063. Curr Biol. 2011. PMID: 22075433 Review.
Cited by
- Functional and Structural Characterization of Bub3·BubR1 Interactions Required for Spindle Assembly Checkpoint Signaling in Human Cells.
Prinz F, Puetter V, Holton SJ, Andres D, Stegmann CM, Kwiatkowski D, Prechtl S, Petersen K, Beckmann G, Kreft B, Mumberg D, Fernández-Montalván A. Prinz F, et al. J Biol Chem. 2016 May 20;291(21):11252-67. doi: 10.1074/jbc.M115.702142. Epub 2016 Mar 30. J Biol Chem. 2016. PMID: 27030009 Free PMC article. - Two functionally distinct kinetochore pools of BubR1 ensure accurate chromosome segregation.
Zhang G, Mendez BL, Sedgwick GG, Nilsson J. Zhang G, et al. Nat Commun. 2016 Jul 26;7:12256. doi: 10.1038/ncomms12256. Nat Commun. 2016. PMID: 27457023 Free PMC article. - Under Tension: Kinetochores and Basic Research.
Biggins S. Biggins S. Genetics. 2015 Jul;200(3):681-2. doi: 10.1534/genetics.115.178467. Genetics. 2015. PMID: 26170442 Free PMC article. - Kinetochore-localized BUB-1/BUB-3 complex promotes anaphase onset in C. elegans.
Kim T, Moyle MW, Lara-Gonzalez P, De Groot C, Oegema K, Desai A. Kim T, et al. J Cell Biol. 2015 May 25;209(4):507-17. doi: 10.1083/jcb.201412035. Epub 2015 May 18. J Cell Biol. 2015. PMID: 25987605 Free PMC article. - Autophosphorylation is sufficient to release Mps1 kinase from native kinetochores.
Koch LB, Opoku KN, Deng Y, Barber A, Littleton AJ, London N, Biggins S, Asbury CL. Koch LB, et al. Proc Natl Acad Sci U S A. 2019 Aug 27;116(35):17355-17360. doi: 10.1073/pnas.1901653116. Epub 2019 Aug 12. Proc Natl Acad Sci U S A. 2019. PMID: 31405987 Free PMC article.
References
- Przewloka MR, Glover DM. The kinetochore and the centromere: a working long distance relationship. Annu Rev Genet. 2009;43:439–465. - PubMed
- Zich J, Hardwick KG. Getting down to the phosphorylated 'nuts and bolts' of spindle checkpoint signalling. Trends Biochem Sci. 2010;35:18–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA015704/CA/NCI NIH HHS/United States
- P50 GM076547/GM/NIGMS NIH HHS/United States
- GM064386/GM/NIGMS NIH HHS/United States
- R01 GM064386/GM/NIGMS NIH HHS/United States
- PM50 GM076547/GM/NIGMS NIH HHS/United States
- T32 CA080416/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials