γ-Aminobutyric acid (GABA) signalling in human pancreatic islets is altered in type 2 diabetes - PubMed (original) (raw)
γ-Aminobutyric acid (GABA) signalling in human pancreatic islets is altered in type 2 diabetes
J Taneera et al. Diabetologia. 2012 Jul.
Abstract
Aims/hypothesis: γ-Aminobutyric acid (GABA) is a signalling molecule in the interstitial space in pancreatic islets. We examined the expression and function of the GABA signalling system components in human pancreatic islets from normoglycaemic and type 2 diabetic individuals.
Methods: Expression of GABA signalling system components was studied by microarray, quantitative PCR analysis, immunohistochemistry and patch-clamp experiments on cells in intact islets. Hormone release was measured from intact islets.
Results: The GABA signalling system was compromised in islets from type 2 diabetic individuals, where the expression of the genes encoding the α1, α2, β2 and β3 GABA(A) channel subunits was downregulated. GABA originating within the islets evoked tonic currents in the cells. The currents were enhanced by pentobarbital and inhibited by the GABA(A) receptor antagonist, SR95531. The effects of SR95531 on hormone release revealed that activation of GABA(A) channels (GABA(A) receptors) decreased both insulin and glucagon secretion. The GABA(B) receptor antagonist, CPG55845, increased insulin release in islets (16.7 mmol/l glucose) from normoglycaemic and type 2 diabetic individuals.
Conclusions/interpretation: Interstitial GABA activates GABA(A) channels and GABA(B) receptors and effectively modulates hormone release in islets from type 2 diabetic and normoglycaemic individuals.
Figures
Fig. 1
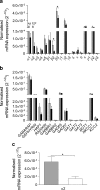
Gene expression of GABA signalling system components in human islets determined by RT-qPCR. a In islets from normoglycaemic donors (grey bar, n = 14) and donors with type 2 diabetes (black bar, n = 9), 18 out of 19 GABAA channel subunits were detected, with the most prominent expression level for α1, α2, β3, γ2, π and ρ2. In islets from individuals with type 2 diabetes, expression of the α1, α2, β2 and β3 GABAA channel subunits was significantly downregulated compared with normoglycaemic donors. b Gene expression of GABA signalling system accessory proteins, receptors, enzymes and transporters from normoglycaemic donors (grey bar, n = 14) and donors with type 2 diabetes (black bar, n = 9). The most prominent expression was detected for GEPHRYN, GABARAP, RADIXIN, GAD65 and the chloride transporter, NKCC1 (also known as SLC12A2). c In islets from hyperglycaemic donors (open bar, n = 6), expression of the α2 GABAA channel subunit was downregulated compared with normoglycaemic donors (grey bar, n = 14). The relative expression of each target gene was normalised to reference gene ACTB using the method. All individual experiments were run in duplicate, and the data presented as mean ± SEM. Differences in expression levels were analysed by Student's t test: *p < 0.05, **p < 0.01, ***p < 0.001
Fig. 2
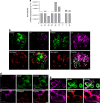
GABAA channel subunit mRNA expression and protein localisation in human pancreatic islet alpha and beta cells. a Expression of GABAA channel subunits in sorted human pancreatic islet beta cells from one normoglycaemic donor quantified by RT-qPCR. The β3 and γ2 subunits were prominently expressed, and the expression of the α1, α2, π and ρ2 subunits was somewhat lower. The relative expression of each target gene was normalised to the reference gene ACTB using the method. b Human islets were co-labelled with antibodies staining insulin (green), α1 GABAA channel subunit (purple) and glucagon (red). Scale bar, 5 μm. c Human islets were co-labelled with antibodies staining insulin (green), α2 GABAA channel subunit (purple) and glucagon (red). Scale bar, 5 μm. d Immunoreactivity of α1 GABAA channel subunit (purple) was detected in both insulin-positive cells (green) and glucagon-positive cells (red) from human islets. Scale bar, 5 μm. e Immunoreactivity of α2 GABAA channel subunit (purple) was detected in both insulin-positive cells (green) and glucagon-positive cells (red) from human islets. Scale bar, 5 μm
Fig. 3
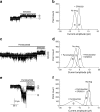
Activation, by interstitial GABA, of GABAA channel currents that are enhanced by pentobarbital in human pancreatic islets. a A representative current trace showing GABA-activated tonic current, recorded from a cell in an intact human islet from a normoglycaemic donor and activated by interstitial GABA, was inhibited by application of SR95531 (100 μmol/l), a GABAA channel antagonist, causing a clear outward shift in the holding current (line 1 to line 2). SR95531 similarly inhibited the baseline current in islet cells in another four experiments. b Gaussian fits to all-point histograms derived from 30 s current recordings in (a) before (1) and during (2) application of SR95531. The difference between the peaks of the Gaussian fits denotes the mean tonic current (−4.4 pA). c A representative current trace showing that pentobarbital (100 μmol/l) increased the GABA-activated tonic current (inward shift of the holding current line 1 to line 3), recorded from a cell in an intact human islet from a normoglycaemic donor and activated by the interstitial GABA. The current was then inhibited by 100 μmol/l SR95531 (outward shift of the holding current line 3 to line 2). Pentobarbital similarly enhanced the GABA-generated current in islet cells in another five experiments. d Gaussian fits to all-point histograms derived from 30 s current recordings in (c) before (1) and during application of pentobarbital (3) or pentobarbital together with SR95531 (2). The peak of the Gaussian fits denotes the mean holding currents (1, −3.6 pA; 2, 0 pA; 3, −7.1 pA). e A representative current trace showing that pentobarbital (100 μmol/l) increased the GABA-activated tonic current (inward shift of the holding current line 1 to line 3), recorded from a cell in an intact human islet from a type 2 diabetes donor and activated by the interstitial GABA. The current was then partially inhibited by 100 μmol/l SR95531 (outward shift of the holding current line 3 to line 2). f. Gaussian fits to all-point histograms derived from 30 s current recordings in (e) before (1) and during application of pentobarbital (3) or pentobarbital together with SR95531 (2). The peak of the Gaussian fits denotes the mean holding currents (1, 0 pA; 2, −2.1 pA; 3, −16.1 pA). Currents were recorded in 20 mmol/l glucose from cells in intact islets at the holding potential of −90 mV in the whole-cell patch-clamp configuration. As no GABA was added experimentally, the GABA must have originated within the islet
Fig. 4
Interstitial GABA activates single-channel currents in intact islets. Single-channel currents that were later inhibited by 100 μmol/l SR95531 were recorded in three different cells (a, b, c) from normoglycaemic donors. a A representative current trace (top trace, slow time scale, s) showing GABA-activated single-channel currents and inhibition by 100 μmol/l SR95531. The solid line shows the time when the extracellular solution containing SR95531 was perfused through the recording chamber. The broken lines indicate from where in the recording the current trace on the faster time scale (ms) was obtained. The glucose concentration was 20 mmol/l and the holding potential was −90 mV (a), −70 mV (b) and −70 mV (c). The most prominent single-channel conductance in the cells in a, b and c was 51 pS, 36 pS and 71 pS, respectively. For all three cells, the currents were recorded in the whole-cell patch-clamp configuration from cells in intact islets and were activated by interstitial GABA and inhibited by application of SR95531 (100 μmol/l)
Fig. 5
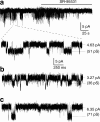
GABAA channel and GABAB receptor antagonists increase hormone release in pancreatic islets from normoglycaemic and type 2 diabetic individuals. a Insulin release was increased in 1 mmol/l glucose (1G) but decreased in 16.7 mmol/l glucose (16.7G) in islets from type 2 diabetic individuals (black bar) compared with those from normoglycaemic individuals (grey bar). SR95531, a GABAA channel antagonist, at a concentration of 10 μmol/l did not modulate insulin release. b Glucagon release was increased in 16.7 but not 1 mmol/l glucose in islets from type 2 diabetic individuals compared with those from normoglycaemic donors. SR95531 (10 μmol/l) increased glucagon release at both glucose concentrations in islets from type 2 diabetic and normoglycaemic individuals. c Insulin release was increased by the GABAB antagonist, CGP55845 (10 μmol/l) in 16.7 but not 1 mmol/l glucose in islets from type 2 diabetic and normoglycaemic individuals. d Glucagon release was not affected by CGP55845 (10 μmol/l). Grey bars: n = 19–31 from four to six normoglycaemic individuals. Black bars: n = 19 from three type 2 diabetic donors. Data are presented as mean ± SEM; *p < 0.05, ***p < 0.001. e Correlation of the α1 GABAA subunit gene expression with insulin secretion measured at 16.7 mmol/l glucose and HbA1c level. A negative correlation with HbA1c (black circles; n = 51; R = 0.3724; p = 0.0071) and no correlation with insulin (grey circles; n = 53; R = 0.1655; p = 0.2363) were observed. Correlation analysis was performed using non-parametric Spearman’s test. f Correlation of the α2 GABAA subunit gene expression with insulin secretion measured at 16.7 mmol/l glucose and HbA1c level. A negative correlation with HbA1c (black circles; n = 51; R = 0.6453; p < 0.0001) and positive correlation with insulin (grey circles; n = 53; R = 0.4790; p = 0.0003) were observed. Correlation analysis was performed using non-parametric Spearman’s test. NB To convert values for HbA1c in % into mmol/mol, subtract 2.15 and multiply by 10.929
References
- Winnock F, Ling Z, De Proft R, et al. Correlation between GABA release from rat islet beta-cells and their metabolic state. Am J Physiol Endocrinol Metab. 2002;282:E937–942. - PubMed
- Pizarro-Delgado J, Braun M, Hernandez-Fisac I, Martin-Del-Rio R, Tamarit-Rodriguez J. Glucose promotion of GABA metabolism contributes to the stimulation of insulin secretion in beta-cells. Biochem J. 2010;431:381–389. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical