Interleukin-1 beta influences on lysyl oxidases and matrix metalloproteinases profile of injured anterior cruciate ligament and medial collateral ligament fibroblasts - PubMed (original) (raw)
Comparative Study
Interleukin-1 beta influences on lysyl oxidases and matrix metalloproteinases profile of injured anterior cruciate ligament and medial collateral ligament fibroblasts
Jing Xie et al. Int Orthop. 2013 Mar.
Abstract
Purpose: The anterior cruciate ligament (ACL) is known to have a poor healing ability, especially in comparison with the medial collateral ligament (MCL) which can heal relatively well. Interleukin-1beta (IL-1β) is considered to be an important chemical mediator in the acute inflammatory phase of ligament injury. The role of IL-1β-induced expressions of lysyl oxidases (LOXs) and matrix metalloproteinases (MMPs), which respectively facilitate extracellular matrix (ECM) repair and degradation, is poorly understood. In this study, we aim to determine the intrinsic differences between ACL and MCL by characterising the differential expressions of LOXs and MMPs in response to IL-1β in the injury process.
Methods: Semi-quantitative polymerase chain reaction (PCR), quantitative real-time PCR, Western blot, and zymography were performed.
Results: We detected high expressions of IL-1β-induced LOXs in normal ACL and MCL. Then, we found IL-1β induced injured MCL to express more LOXs than injured ACL (up to 2.85-fold in LOX, 2.58-fold in LOXL-1, 1.89-fold in LOXL-2, 2.46-fold in LOXL-3 and 2.18-fold in LOXL-4). Meanwhile, we found IL-1β induced injured ACL to express more MMPs than injured MCL (up to 1.72-fold in MMP-1, 1.95-fold in MMP-2, 2.05-fold in MMP-3 and 2.3-fold in MMP-12). The further protein results coincided with gene expressions above.
Conclusions: Lower expressions of LOXs and higher expressions of MMPs might help to explain the poor healing ability of ACL.
Figures
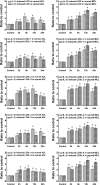
Fig. 1
IL-1β induced dose-dependent increases of LOXs genes in both ACL and MCL fibroblasts. a Semi-quantitative PCR showed IL-1β induced higher gene expressions of LOXs in MCL than those in ACL after 2-h IL-1β treatments. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the reference gene. The gels shown were representative of four different experiments (n = 4); ACL and MCL fibroblasts in the comparison model of the experiments came from the same donors. Cont control, 1 ng, 5 ng and 20 ng 1, 5 and 20 ng/ml IL-1β, respectively. b Quantitative real-time PCR confirmed the different increases of LOXs in both ACL and MCL after 2-h IL-1β treatments. GAPDH was used as the reference gene. The △Ct method was used for measuring the fold changes. 1 ng, 5 ng and 20 ng concentrations of 1, 5 and 20 ng/ml IL-1β. The data presented were the mean of five different experiments (n = 5); scale bars SD. *p < 0.05 vs non-treated control
Fig. 2
An amount of 5 ng/ml IL-1β induced different high expressions of LOXs in normal and injured ACL/MCL fibroblasts by quantitative real-time PCR. After Fig. 1 experiments with different concentrations of IL-1β, we chose 5 ng/ml IL-1β to show the LOX family gene variations at the different time points following treatments. We collected samples at 0 (control), 2, 6, 12 and 24 h after 5 ng/ml IL-1β treatments in normal and injured ACL/MCL fibroblasts. GAPDH was used as the reference gene. The △Ct method was used for measuring the fold changes. The data presented were the mean of four different experiments (n = 4). *Significant difference with respect to control (p < 0.05)
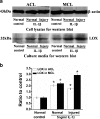
Fig. 3
IL-1β promoted protein expressions of LOX in normal and injured ACL/MCL fibroblasts. a Western blot showed LOX expressions in normal and injured ACL/MCL fibroblasts after being treated with 5 ng/ml IL-1β. The blot gels shown are representative of four different experiments (n = 4). The culture media samples were collected at 72 h following treatments for LOX expressions and the cell lysates samples for β-actin. b Quantitative analysis of the Western blot with Bio-Rad image software (Quantity One 4.6.3 software). The data were the mean of four different experiments (n = 4); SD. *Significant difference with respect to control (p < 0.05)
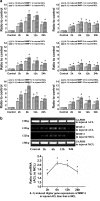
Fig. 4
IL-1β induced higher gene expressions of MMPs in injured ACL than those in MCL fibroblasts. a Quantitative real-time PCR showed higher gene expressions of MMP-1, -2 and -12 in injured ACL than those in MCL fibroblasts. The data were recorded after 0 (control), 2, 6, 12 and 24 h following 5 ng/ml IL-1β treatments. The data for each sample were normalised to GAPDH mRNA. Data (means ± SD, n = 4) were represented as the fold change in expression compared to control. *p < 0.05. b The mRNA of MMP-3 in ACL and MCL cells were too low to detect using quantitative real-time PCR by the △Ct method loading 1 μl cDNA coordinate with other MMPs. Through GAPDH using semi-quantitative PCR with 38 cycles, increased gene expressions reached value peaks at 6 h after 5 ng/ml IL-1β in injured ACL and MCL. Notably, the ratio of MMP-3 mRNA (injured ACL to MCL) was high. The data were the mean of four different experiments (n = 4). *sSgnificant difference with respect to control (p < 0.05)
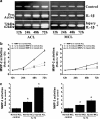
Fig. 5
IL-1β induced higher activities of MMP-2 in injured ACL than those in injured MCL fibroblasts. a Zymography showed different expressions of MMP-2 in normal and injured ACL/MCL fibroblasts. The gels shown were representative of four different experiments (n = 4). b Quantification of MMP-2 activities showed time-dependent increases of MMP-2 activities in both normal and injured ACL/MCL. Quantification was done with Quantity One 4.6.3 software. Optical densities of the pro-MMP-2 and active-MMP-2 bands were added as the total value of activity for MMP-2. Then, the values of 24, 48 and 72 h were compared to the values of 12 h. c The indicated quantitative data refer to 72-h time points of control and treated groups, respectively. Besides, the band 62 kDa active form MMP-2 was calculated as 10 times density of the 72 kDa pro-MMP-2 band as described previously [5, 8]. The data were the mean of four different experiments (n = 4). *Significant difference with respect to control (p < 0.05)
Similar articles
- Influences of Tumor Necrosis Factor-α on Lysyl Oxidases and Matrix Metalloproteinases of Injured Anterior Cruciate Ligament and Medial Collateral Ligament Fibroblasts.
Cai L, An S, Liao J, Yang W, Zhou X, Sung KL, Xie J. Cai L, et al. J Knee Surg. 2017 Jan;30(1):78-87. doi: 10.1055/s-0036-1581135. Epub 2016 Apr 18. J Knee Surg. 2017. PMID: 27088365 - TGF-beta1 induces the different expressions of lysyl oxidases and matrix metalloproteinases in anterior cruciate ligament and medial collateral ligament fibroblasts after mechanical injury.
Xie J, Wang C, Huang DY, Zhang Y, Xu J, Kolesnikov SS, Sung KL, Zhao H. Xie J, et al. J Biomech. 2013 Mar 15;46(5):890-8. doi: 10.1016/j.jbiomech.2012.12.019. Epub 2013 Jan 26. J Biomech. 2013. PMID: 23357697 Clinical Trial. - Differential expressions of lysyl oxidase family in ACL and MCL fibroblasts after mechanical injury.
Xie J, Huang W, Jiang J, Zhang Y, Xu Y, Xu C, Yang L, Chen PC, Sung KL. Xie J, et al. Injury. 2013 Jul;44(7):893-900. doi: 10.1016/j.injury.2012.08.046. Epub 2012 Sep 23. Injury. 2013. PMID: 23010071 - Anterior cruciate ligament and medial collateral ligament injuries.
Bollier M, Smith PA. Bollier M, et al. J Knee Surg. 2014 Oct;27(5):359-68. doi: 10.1055/s-0034-1381961. Epub 2014 Jun 20. J Knee Surg. 2014. PMID: 24949985 Review. - Surgical Management and Treatment of the Anterior Cruciate Ligament/Medial Collateral Ligament Injured Knee.
Dale KM, Bailey JR, Moorman CT 3rd. Dale KM, et al. Clin Sports Med. 2017 Jan;36(1):87-103. doi: 10.1016/j.csm.2016.08.005. Epub 2016 Oct 15. Clin Sports Med. 2017. PMID: 27871663 Review.
Cited by
- Advanced Gene Therapy Strategies for the Repair of ACL Injuries.
Amini M, Venkatesan JK, Liu W, Leroux A, Nguyen TN, Madry H, Migonney V, Cucchiarini M. Amini M, et al. Int J Mol Sci. 2022 Nov 21;23(22):14467. doi: 10.3390/ijms232214467. Int J Mol Sci. 2022. PMID: 36430947 Free PMC article. Review. - Bio-enhanced repair of the anterior cruciate ligament.
Proffen BL, Sieker JT, Murray MM. Proffen BL, et al. Arthroscopy. 2015 May;31(5):990-7. doi: 10.1016/j.arthro.2014.11.016. Epub 2015 Jan 14. Arthroscopy. 2015. PMID: 25595694 Free PMC article. - Influence of TNF-α and biomechanical stress on matrix metalloproteinases and lysyl oxidases expressions in human knee synovial fibroblasts.
Zhang Y, Huang W, Jiang J, Xie J, Xu C, Wang C, Yin L, Yang L, Zhou K, Chen P, Sung KP. Zhang Y, et al. Knee Surg Sports Traumatol Arthrosc. 2014 Sep;22(9):1997-2006. doi: 10.1007/s00167-013-2425-z. Epub 2013 Feb 2. Knee Surg Sports Traumatol Arthrosc. 2014. PMID: 23377799 - Lysyl Oxidase and the Tumor Microenvironment.
Wang TH, Hsia SM, Shieh TM. Wang TH, et al. Int J Mol Sci. 2016 Dec 29;18(1):62. doi: 10.3390/ijms18010062. Int J Mol Sci. 2016. PMID: 28036074 Free PMC article. Review. - Basic science of anterior cruciate ligament injury and repair.
Kiapour AM, Murray MM. Kiapour AM, et al. Bone Joint Res. 2014 Feb 4;3(2):20-31. doi: 10.1302/2046-3758.32.2000241. Print 2014. Bone Joint Res. 2014. PMID: 24497504 Free PMC article.
References
- Kannus P. Long-term results of conservatively treated medial collateral ligament injuries of the knee joint. Clin Orthop Relat Res. 1988;226:103–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous