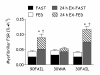Nutritional regulation of muscle protein synthesis with resistance exercise: strategies to enhance anabolism - PubMed (original) (raw)
Nutritional regulation of muscle protein synthesis with resistance exercise: strategies to enhance anabolism
Tyler A Churchward-Venne et al. Nutr Metab (Lond). 2012.
Abstract
Provision of dietary amino acids increases skeletal muscle protein synthesis (MPS), an effect that is enhanced by prior resistance exercise. As a fundamentally necessary process in the enhancement of muscle mass, strategies to enhance rates of MPS would be beneficial in the development of interventions aimed at increasing skeletal muscle mass particularly when combined with chronic resistance exercise. The purpose of this review article is to provide an update on current findings regarding the nutritional regulation of MPS and highlight nutrition based strategies that may serve to maximize skeletal muscle protein anabolism with resistance exercise. Such factors include timing of protein intake, dietary protein type, the role of leucine as a key anabolic amino acid, and the impact of other macronutrients (i.e. carbohydrate) on the regulation of MPS after resistance exercise. We contend that nutritional strategies that serve to maximally stimulate MPS may be useful in the development of nutrition and exercise based interventions aimed at enhancing skeletal muscle mass which may be of interest to elderly populations and to athletes.
Figures
Figure 1
Resistance exercise stimulates a prolonged elevation of muscle protein synthesis (MPS) that can remain elevated for ≥ 24 h (dashed lines). Thus, we propose that protein ingestion at any point during this enhanced period of ‘anabolic potential’ will be additive to these already elevated exercise mediated rates (solid line).
Figure 2
Enhanced amino acid sensitivity of myofibrillar protein synthesis (FSR) persists for up to 24 h only after resistance exercise that results in maximal muscle fibre activation induced by high load low volume resistance exercise (90FAIL) or low load high volume resistance exercise (30FAIL). 30WM represents a worked-match control to the 90FAIL condition that did not result in full muscle fibre recruitment. The change in myofibrillar protein synthesis rates are determined from the transition from fasting (FAST) to feeding 15 g of protein at rest (FED) or 24–27 h after resistance exercise in the fasting- (24 h EX FAST) or fed-state (24 h EX-FED). *Significantly different from FED (P < 0.05). †Significantly different from 30WM (P < 0.05). Adptated from Burd and colleagues [27].
Similar articles
- Dietary proteins and amino acids in the control of the muscle mass during immobilization and aging: role of the MPS response.
Cholewa JM, Dardevet D, Lima-Soares F, de Araújo Pessôa K, Oliveira PH, Dos Santos Pinho JR, Nicastro H, Xia Z, Cabido CE, Zanchi NE. Cholewa JM, et al. Amino Acids. 2017 May;49(5):811-820. doi: 10.1007/s00726-017-2390-9. Epub 2017 Feb 7. Amino Acids. 2017. PMID: 28175999 Review. - International society of sports nutrition position stand: nutrient timing.
Kerksick CM, Arent S, Schoenfeld BJ, Stout JR, Campbell B, Wilborn CD, Taylor L, Kalman D, Smith-Ryan AE, Kreider RB, Willoughby D, Arciero PJ, VanDusseldorp TA, Ormsbee MJ, Wildman R, Greenwood M, Ziegenfuss TN, Aragon AA, Antonio J. Kerksick CM, et al. J Int Soc Sports Nutr. 2017 Aug 29;14:33. doi: 10.1186/s12970-017-0189-4. eCollection 2017. J Int Soc Sports Nutr. 2017. PMID: 28919842 Free PMC article. Review. - International Society of Sports Nutrition Position Stand: protein and exercise.
Jäger R, Kerksick CM, Campbell BI, Cribb PJ, Wells SD, Skwiat TM, Purpura M, Ziegenfuss TN, Ferrando AA, Arent SM, Smith-Ryan AE, Stout JR, Arciero PJ, Ormsbee MJ, Taylor LW, Wilborn CD, Kalman DS, Kreider RB, Willoughby DS, Hoffman JR, Krzykowski JL, Antonio J. Jäger R, et al. J Int Soc Sports Nutr. 2017 Jun 20;14:20. doi: 10.1186/s12970-017-0177-8. eCollection 2017. J Int Soc Sports Nutr. 2017. PMID: 28642676 Free PMC article. Review. - Dietary protein to support anabolism with resistance exercise in young men.
Phillips SM, Hartman JW, Wilkinson SB. Phillips SM, et al. J Am Coll Nutr. 2005 Apr;24(2):134S-139S. doi: 10.1080/07315724.2005.10719454. J Am Coll Nutr. 2005. PMID: 15798080 Review. - Nutrient interaction for optimal protein anabolism in resistance exercise.
Breen L, Phillips SM. Breen L, et al. Curr Opin Clin Nutr Metab Care. 2012 May;15(3):226-32. doi: 10.1097/MCO.0b013e3283516850. Curr Opin Clin Nutr Metab Care. 2012. PMID: 22366920 Review.
Cited by
- Hydrolyzed casein and whey protein meals comparably stimulate net whole-body protein synthesis in COPD patients with nutritional depletion without an additional effect of leucine co-ingestion.
Jonker R, Deutz NE, Erbland ML, Anderson PJ, Engelen MP. Jonker R, et al. Clin Nutr. 2014 Apr;33(2):211-20. doi: 10.1016/j.clnu.2013.06.014. Epub 2013 Jul 1. Clin Nutr. 2014. PMID: 23886411 Free PMC article. Clinical Trial. - Current Concepts and Unresolved Questions in Dietary Protein Requirements and Supplements in Adults.
Phillips SM. Phillips SM. Front Nutr. 2017 May 8;4:13. doi: 10.3389/fnut.2017.00013. eCollection 2017. Front Nutr. 2017. PMID: 28534027 Free PMC article. Review. - High-Protein Foods and Physical Activity Protect Against Age-Related Muscle Loss and Functional Decline.
Bradlee ML, Mustafa J, Singer MR, Moore LL. Bradlee ML, et al. J Gerontol A Biol Sci Med Sci. 2017 Dec 12;73(1):88-94. doi: 10.1093/gerona/glx070. J Gerontol A Biol Sci Med Sci. 2017. PMID: 28549098 Free PMC article. - The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein.
Macnaughton LS, Wardle SL, Witard OC, McGlory C, Hamilton DL, Jeromson S, Lawrence CE, Wallis GA, Tipton KD. Macnaughton LS, et al. Physiol Rep. 2016 Aug;4(15):e12893. doi: 10.14814/phy2.12893. Physiol Rep. 2016. PMID: 27511985 Free PMC article. - Investigating the Combined Effects of Mechanical Stress and Nutrition on Muscle Hypertrophic Signals Using Contractile 3D-Engineered Muscle (3D-EM).
Yi D, Sugimoto T, Matsumura T, Yokoyama S, Fujisato T, Nakamura T, Hashimoto T. Yi D, et al. Nutrients. 2023 Sep 21;15(18):4083. doi: 10.3390/nu15184083. Nutrients. 2023. PMID: 37764867 Free PMC article.
References
- Burd NA, Tang JE, Moore DR, Phillips SM. Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences. J Appl Physiol. 2009;106:1692–1701. - PubMed
- Kumar V, Atherton P, Smith K, Rennie MJ. Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol. 2009;106:2026–2039. - PubMed
- Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997;273:E122–E129. - PubMed
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2005;19:422–424. - PubMed
- Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009;89:161–168. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials