FXR signaling in the enterohepatic system - PubMed (original) (raw)
Review
FXR signaling in the enterohepatic system
Tsutomu Matsubara et al. Mol Cell Endocrinol. 2013.
Abstract
Enterohepatic circulation serves to capture bile acids and other steroid metabolites produced in the liver and secreted to the intestine, for reabsorption back into the circulation and reuptake to the liver. This process is under tight regulation by nuclear receptor signaling. Bile acids, produced from cholesterol, can alter gene expression in the liver and small intestine via activating the nuclear receptors farnesoid X receptor (FXR; NR1H4), pregnane X receptor (PXR; NR1I2), vitamin D receptor (VDR; NR1I1), G protein coupled receptor TGR5, and other cell signaling pathways (JNK1/2, AKT and ERK1/2). Among these controls, FXR is known to be a major bile acid-responsive ligand-activated transcription factor and a crucial control element for maintaining bile acid homeostasis. FXR has a high affinity for several major endogenous bile acids, notably cholic acid, deoxycholic acid, chenodeoxycholic acid, and lithocholic acid. By responding to excess bile acids, FXR is a bridge between the liver and small intestine to control bile acid levels and regulate bile acid synthesis and enterohepatic flow. FXR is highly expressed in the liver and gut, relative to other tissues, and contributes to the maintenance of cholesterol/bile acid homeostasis by regulating a variety of metabolic enzymes and transporters. FXR activation also affects lipid and glucose metabolism, and can influence drug metabolism.
Published by Elsevier Ireland Ltd.
Figures
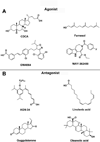
Figure 1. Reported FXR ligands
(A) FXR agonists, CDCA (steroid), farnesol (terpenoid), GW4064 (aromatics), and WAY-362450 (alkaloid). (B) FXR antagonists, AGN-34 (aromatics), linolenic acid (fatty acid), guggulsterone (steroid), oleanoic acid (terpenoid).
Figure 2. Metabolomic analysis of CA-induced and untreated wild-type (+/+) and Fxr-null (−/−) mice by ultraperformance™ liquid chromatography electrospray ionization quadrupole time-of-flight mass spectrometry
(A) Scores scatter plot of principal components analysis (PCA) model of urine from the control and CA-treated groups in both wild-type (+/+) and _Fxr_null (−/−) mice. (B) The adaptive metabolic pathways of _Fxr_-null mice upon CA challenge. Under the effect of bile acid-CoA:amino acid _N_-acyltransferase (BAAT) and UDP-glucuronosyltransferase (UGT), cholic acid (CA) is converted to taurocholate and cholate glucoside. Taurocholate can be further metabolized into taurocholate glucoside and tauro-3α,6,7α,12α-tetrol under the effect of glucosyl-transferase and cytochromes P450, respectively. The level of inflammatory cytokines TNFα and TGFβ increase in _Fxr_-null mice upon CA challenge, which enhances the level of corticosterone in vivo. Under the cytochromes P450 catalysis and 21-hydroxysteroid dehydrogenase (HSD), corticosterone is transformed to HDOPA. HDOPA can further be converted to DHOPA and hydroxy-HDOPA under by cytochrome P450. Hydroxy-DHOPA is generated from DHOPA.
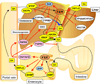
Figure 3
Major roles of FXR signaling in the enterohepatic system. FXR accelerates bile acid export from liver through induction of BSEP and ABCB4 expression and decreasing bile acid uptake to liver by the suppression of NTCP and OATPs expression via hepatic FXR-SHP signaling, decreases bile acid absorption at the intestine through suppression of ASBT via FXR-SHP signaling, and attenuates cholesterol metabolism/bile acid synthesis by suppression of CYP7A1 and CYP8B1 expression via the hepatic FXR-SHP and intestinal FGF15/19 pathways. Thus, FXR action leads to decreased bile acid pool size. Intestinal FXR-FGF15/19 signaling decreases hepatic glucose metabolism through FGFR4 and induces gallbladder filling through FGFR3.
Similar articles
- Bile acids are nutrient signaling hormones.
Zhou H, Hylemon PB. Zhou H, et al. Steroids. 2014 Aug;86:62-8. doi: 10.1016/j.steroids.2014.04.016. Epub 2014 May 10. Steroids. 2014. PMID: 24819989 Free PMC article. Review. - Bile acids as metabolic regulators.
Li T, Chiang JY. Li T, et al. Curr Opin Gastroenterol. 2015 Mar;31(2):159-65. doi: 10.1097/MOG.0000000000000156. Curr Opin Gastroenterol. 2015. PMID: 25584736 Free PMC article. Review. - Nuclear receptor control of enterohepatic circulation.
Gonzalez FJ. Gonzalez FJ. Compr Physiol. 2012 Oct;2(4):2811-28. doi: 10.1002/cphy.c120007. Compr Physiol. 2012. PMID: 23720266 Free PMC article. Review. - Bile acids as regulators of hepatic lipid and glucose metabolism.
Trauner M, Claudel T, Fickert P, Moustafa T, Wagner M. Trauner M, et al. Dig Dis. 2010;28(1):220-4. doi: 10.1159/000282091. Epub 2010 May 7. Dig Dis. 2010. PMID: 20460915 Review.
Cited by
- Mouse models of nonalcoholic steatohepatitis and their application to new drug development.
Phung HH, Lee CH. Phung HH, et al. Arch Pharm Res. 2022 Nov;45(11):761-794. doi: 10.1007/s12272-022-01410-5. Epub 2022 Nov 1. Arch Pharm Res. 2022. PMID: 36318445 Review. - Exposure of Ldlr-/- Mice to a PFAS Mixture and Outcomes Related to Circulating Lipids, Bile Acid Excretion, and the Intestinal Transporter ASBT.
Roth K, Yang Z, Agarwal M, Birbeck J, Westrick J, Lydic T, Gurdziel K, Petriello MC. Roth K, et al. Environ Health Perspect. 2024 Aug;132(8):87007. doi: 10.1289/EHP14339. Epub 2024 Aug 23. Environ Health Perspect. 2024. PMID: 39177951 Free PMC article. - An Intestinal Microbiota-Farnesoid X Receptor Axis Modulates Metabolic Disease.
Gonzalez FJ, Jiang C, Patterson AD. Gonzalez FJ, et al. Gastroenterology. 2016 Nov;151(5):845-859. doi: 10.1053/j.gastro.2016.08.057. Epub 2016 Sep 14. Gastroenterology. 2016. PMID: 27639801 Free PMC article. Review. - Bile acid transporter-mediated oral drug delivery.
Deng F, Bae YH. Deng F, et al. J Control Release. 2020 Nov 10;327:100-116. doi: 10.1016/j.jconrel.2020.07.034. Epub 2020 Jul 22. J Control Release. 2020. PMID: 32711025 Free PMC article. Review. - Electroacupuncture Improves Insulin Resistance in Type 2 Diabetes Mice by Regulating Intestinal Flora and Bile Acid.
Pan T, Li X, Guo X, Wang H, Zhou X, Shang R, Xie D, Qian X, Dai M, Fan E, Chen X, Chen C. Pan T, et al. Diabetes Metab Syndr Obes. 2023 Dec 7;16:4025-4042. doi: 10.2147/DMSO.S421134. eCollection 2023. Diabetes Metab Syndr Obes. 2023. PMID: 38089431 Free PMC article.
References
- Seol W, Choi HS, Moore DD. Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors. Mol Endocrinol. 1995;9:72–85. - PubMed
- Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, Evans RM, Weinberger C. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. - PubMed
- Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. - PubMed
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. - PubMed
- Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous