Analysis of factors affecting Ca(2+)-dependent inactivation dynamics of L-type Ca(2+) current of cardiac myocytes in pulmonary vein of rabbit - PubMed (original) (raw)
Analysis of factors affecting Ca(2+)-dependent inactivation dynamics of L-type Ca(2+) current of cardiac myocytes in pulmonary vein of rabbit
Ju Seok Ryu et al. J Physiol. 2012.
Abstract
L-type Ca(2+) channels (ICaLs) are inactivated by an increase in intracellular [Ca(2+)], known as Ca(2+)-dependent inactivation (CDI). CDI is also induced by Ca(2+) released from the sarcoplasmic reticulum (SR), known as release-dependent inhibition (RDI). As both CDI and RDI occur in the junctional subsarcolemmal nanospace (JSS), we investigated which factors are involved within the JSS using isolated cardiac myocytes from the main pulmonary vein of the rabbit. Using the whole-cell patch clamp technique, RDI was readily observed with the application of a pre-pulse followed by a test pulse, during which the ICaLs exhibited a decrease in peak current amplitude and a slower inactivation. A fast acting Ca(2+) chelator, 1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA), abolished this effect. As the time interval between the pre-pulse and test pulse increased, the ICaLs exhibited greater recovery and the RDI was relieved. Inhibition of the ryanodine receptor (RyR) or the SR Ca(2+)-ATPase (SERCA) greatly attenuated RDI and facilitated ICaL recovery. Removal of extracellular Na(+),which inhibits the Na(+)-Ca(2+) exchange (Incx), greatly enhanced RDI and slowed ICaL recovery, suggesting that Incx critically controls the [Ca(2+)] in the JSS. We incorporated the Ca(2+)-binding kinetics of the ICaL into a previously published computational model. By assuming two Ca(2+)-binding sites in the ICaL, of which one is of low-affinity with fast kinetics and the other is of high-affinity with slower kinetics, the new model was able to successfully reproduce RDI and its regulation by Incx. The model suggests that Incx accelerates Ca(2+) removal from the JSS to downregulate CDI and attenuates SR Ca(2+) refilling. The model may be useful to elucidate complex mechanisms involved in excitation–contraction coupling in myocytes.
Figures
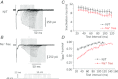
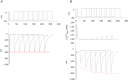
Figure 1. Inactivation of ICaLs with 0.1 mm EGTA (A) or 10 mm BAPTA (B) as the Ca2+ chelator
The rate of inactivation was much slower with BAPTA than with EGTA.
Figure 2. Characteristics of release-dependent inactivation (RDI)
A, repetitive step pulses separated by 30 s intervals did not induce any change in the ICaLs. B, when a pre-pulse was applied, the inward current activated by Ca2+ appeared, after which RDI of the ICaLs was observed with a reduced peak current and slower rate of inactivation.
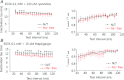
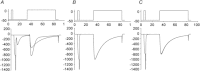
Figure 3. Effect of altering the change in intracellular [Ca2+] on release-dependent inactivation (RDI)
A, suppression of the intracellular [Ca2+] change by 10 m
m
BAPTA eliminated the RDI. B, suppression of Ca2+ release from the SR by ryanodine caused a significant decrease in the RDI.
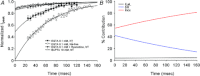
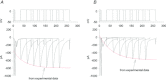
Figure 4. Effects of increasing the time interval between pre-pulse and test pulse on the extent of RDI
The traces from subsequent experiments are overlapped to illustrate the trend. A, the amplitude of the ICaL current initially decreased as a result of the pre-pulse; as the time interval between the pre-pulse and test pulse increased, the amplitude of the ICaL current recovered to that of the control, and the inactivation rate also increased. B, under conditions of Na+ deprivation (Na+-free solution), the current amplitude was smaller and recovery was slower than in normal physiological (NT) solution. C, the inactivation time constant for the ICaL was always less in the Na+-free solution than in NT solution, meaning the rate of inactivation of ICaL was faster in the absence of Na+. D, the decrease in current amplitude attributable to RDI was larger and recovered more slowly in Na+-free solution than in NT solution.
Figure 5. Effect of fast Ca2+ chelation by BAPTA on the time-dependent change in release-dependent inactivation (RDI)
A and B, with the intracellular [Ca2+] change suppressed by 10 m
m
BAPTA, the L-type Ca2+ channel (ICaL) was little changed, as seen by a series of step pulses, regardless of the presence (A) or absence (B) of extracellular Na+. C, the inactivation time constant of the ICaL was unchanged. D, the amplitude of the ICaL current was slightly decreased when the time interval between the pre-pulse and test pulse was less than 20 ms; this effect may be due to voltage-dependent inactivation (see text).
Figure 6. Time-dependent changes in release-dependent inactivation (RDI) when SR function is suppressed
A, in the presence of 150 μ
m
ryanodine, the peak current amplitude was little changed, similar to the pattern seen in the presence of BAPTA (see Fig. 5_C_), although the inactivation time constant was smaller and decreased slightly as the time interval between the pre-pulse and test pulse increased. Removal of extracellular Na+ decreased the inactivation time further. B, similar findings were observed in the presence of 10 μ
m
thapsigargin.
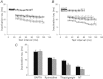
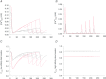
Figure 7. Influence of each factor on time-dependent changes in the peak current of L-type Ca2+ channels (ICaLs)
A, the current amplitudes were normalised to the control amplitude. The _X_-axis denoted the time interval between the pre-pulse and the test pulse. Extrapolating to zero time between pre-pulse and test pulse, in BAPTA accessible space, the relative contributions of ICaLs, SR, and inhibition of Incx to the decrease of peak current were approximately 4%, 42% and 54%, respectively. B, relative contributions of the ICaLs (black), SR (blue), and inhibition of Incx (red) to the decrease of ICaL current as a function of the time interval between the pre-pulse and test pulse.
Figure 8. Influence of each factor on the inactivation time constant
A, under physiological (NT) conditions, the changes in the inactivation time constant as a function of the time interval between the pre-pulse and test pulse in the presence of BAPTA (•), ryanodine (○), thapsigargin (▾) and none (▵). B, under Na+-free conditions, the changes in the inactivation time constant in the presence of BAPTA (•), ryanodine (○), thapsigargin (▾) and none (▵). C, the control inactivation time constants obtained by the single pulse applied at every 30 s. Black bars denotes NT conditions and grey bars show Na+-free conditions. Those were the paired experiments and ** denoted P value < 0.01.
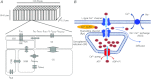
Figure 9. Model structure of cardiac myocytes in the pulmonary vein, showing components involved with the change of Ca2+ concentration in the junctional subsarcolemmal space (JSS)
A, the diagram shows half a sarcomere (adapted from Leem et al. 2006). B, sources of Ca2+ ingress and egress in the JSS.
Figure 10. Results from a model simulation of release-dependent inactivation (RDI)
The model simulation satisfactorily reproduced the experimental measurements of RDI in the presence of 0.1 m
m
EGTA/NT (A), 10 m
m
BAPTA/NT (B), and 0.1 m
m
EGTA/Ryanodine (C) (see text). Top, depiction of the voltage pulse train given to the L-type Ca2+ channels (ICaLs). Bottom, simulated ICaL currents resulting from the voltage pulses.
Figure 11. Model simulation of the recovery from release-dependent inactivation (RDI)
The model simulation satisfactorily reproduced the experimental measurements of RDI recovery in the presence of 0.1 m
m
EGTA under physiological conditions (A), and 0.1 m
m
EGTA in Na+-free medium (B). Top, depiction of the voltage pulse train given to the L-type Ca2+ channels (ICaLs), with varying times between the pre-pulse and test pulse. Bottom, simulated ICaL currents resulting from the voltage pulses. The traces from separate simulations are overlapped to show the trend. Red lines are from the experimental data.
Figure 12. Model simulations of the Ca2+ concentration in the junctional subsarcolemmal (A) region ([Ca2+]JSR) or (B) space ([Ca2+]JSS), and (C and D) the corresponding L-type Ca2+ channel (ICaL) current due to Ca2+ binding at two different types of Ca2+-binding sites in a series of double-pulse experiments (see text)
The traces from several simulations at different time intervals between the pre-pulse and test pulse are overlapped to show the trends. A, before the pre-pulse, the Ca2+ content of the SR was greater in the Na+-free medium (red) than under physiological (NT) conditions (black) because Ca2+ refilling was facilitated in the absence of Na+. B, the Ca2+ released from the SR during the pre-pulse and subsequent pulses generated a much larger increase in [Ca2+]JSS near the ICaL when Na+-free medium (red) was used as compared with NT conditions (black). C, the higher [Ca2+]JSS in the absence of Na+ (red) as compared with NT conditions (black) decreased the open probability of LAFK sites with bound Ca2+, leading to lesser ICaL activity. D, the HASK Ca2+-binding site contributed less to the overall ICaL activity because the slow kinetics but determined the availability of ICaLs. Black line: NT, Red line: Na+-free.
Figure 13. Model simulation of release-dependent inactivation (RDI) under specific conditions
A, with the SR function removed, RDI did not occur under Na+-free conditions, indicating that Na+–Ca2+ exchange (Incx) had virtually no role in the absence of an active SR. B, with an active SR, the RDI in the absence of Na+ (inactive Incx) is greater than that under physiological conditions (active Incx). Top, depiction of the voltage pulse train given to the L-type Ca2+ channels (ICaLs), with varying times between the pre-pulse and test pulse. Middle (B), simulated [Ca2+] in the junctional subsarcolemmal space. Bottom, simulated ICaL currents resulting from the voltage pulses. The traces from separate simulations are overlapped to show the trends. Red lines are the experimental results from control ICaL current amplitude measurements during RDI.
Similar articles
- Microdomain [Ca²⁺] near ryanodine receptors as reported by L-type Ca²⁺ and Na+/Ca²⁺ exchange currents.
Acsai K, Antoons G, Livshitz L, Rudy Y, Sipido KR. Acsai K, et al. J Physiol. 2011 May 15;589(Pt 10):2569-83. doi: 10.1113/jphysiol.2010.202663. Epub 2011 Mar 8. J Physiol. 2011. PMID: 21486798 Free PMC article. - Theoretical study of L-type Ca(2+) current inactivation kinetics during action potential repolarization and early afterdepolarizations.
Morotti S, Grandi E, Summa A, Ginsburg KS, Bers DM. Morotti S, et al. J Physiol. 2012 Sep 15;590(18):4465-81. doi: 10.1113/jphysiol.2012.231886. Epub 2012 May 14. J Physiol. 2012. PMID: 22586219 Free PMC article. - Calmodulin kinase II accelerates L-type Ca2+ current recovery from inactivation and compensates for the direct inhibitory effect of [Ca2+]i in rat ventricular myocytes.
Guo J, Duff HJ. Guo J, et al. J Physiol. 2006 Jul 15;574(Pt 2):509-18. doi: 10.1113/jphysiol.2006.109199. Epub 2006 Apr 20. J Physiol. 2006. PMID: 16627565 Free PMC article. - Inactivation of L-type calcium channels in cardiomyocytes. Experimental and theoretical approaches.
Kubalová Z. Kubalová Z. Gen Physiol Biophys. 2003 Dec;22(4):441-54. Gen Physiol Biophys. 2003. PMID: 15113117 Review.
Cited by
- What can modelling provide to cardiac physiology?
Smith NP, McCulloch AD, Paterson DJ. Smith NP, et al. J Physiol. 2012 Sep 15;590(18):4401-2. doi: 10.1113/jphysiol.2012.242578. J Physiol. 2012. PMID: 22988258 Free PMC article. No abstract available. - Impaired Inactivation of L-Type Ca2+ Current as a Potential Mechanism for Variable Arrhythmogenic Liability of HERG K+ Channel Blocking Drugs.
Kim JG, Sung DJ, Kim HJ, Park SW, Won KJ, Kim B, Shin HC, Kim KS, Leem CH, Zhang YH, Cho H, Bae YM. Kim JG, et al. PLoS One. 2016 Mar 1;11(3):e0149198. doi: 10.1371/journal.pone.0149198. eCollection 2016. PLoS One. 2016. PMID: 26930604 Free PMC article. - L-type Ca2+ channel recovery from inactivation in rabbit atrial myocytes.
Martinez-Hernandez E, Blatter LA, Kanaporis G. Martinez-Hernandez E, et al. Physiol Rep. 2022 Mar;10(5):e15222. doi: 10.14814/phy2.15222. Physiol Rep. 2022. PMID: 35274829 Free PMC article.
References
- Anderson ME. Ca2+-dependent regulation of cardiac L-type Ca2+ channels: is a unifying mechanism at hand? J Mol Cell Cardiol. 2001;33:639–650. - PubMed
- Balke CW, Wier WG. Ryanodine does not affect calcium current in guinea pig ventricular myocytes in which Ca2+ is buffered. Circ Res. 1991;68:897–902. - PubMed
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Boston: Kluwer; 2001.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous