Kynurenines in the mammalian brain: when physiology meets pathology - PubMed (original) (raw)
Review
Kynurenines in the mammalian brain: when physiology meets pathology
Robert Schwarcz et al. Nat Rev Neurosci. 2012 Jul.
Abstract
The essential amino acid tryptophan is not only a precursor of serotonin but is also degraded to several other neuroactive compounds, including kynurenic acid, 3-hydroxykynurenine and quinolinic acid. The synthesis of these metabolites is regulated by an enzymatic cascade, known as the kynurenine pathway, that is tightly controlled by the immune system. Dysregulation of this pathway, resulting in hyper-or hypofunction of active metabolites, is associated with neurodegenerative and other neurological disorders, as well as with psychiatric diseases such as depression and schizophrenia. With recently developed pharmacological agents, it is now possible to restore metabolic equilibrium and envisage novel therapeutic interventions.
Figures
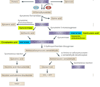
Figure 1. The kynurenine pathway of tryptophan degradation in mammals
The kynurenine pathway is initiated by the transformation of tryptophan to N-formylkynurenine by indoleamine 2,3-dioxygenases (IDO) 1 and 2 , and tryptophan 2,3-dioxygenase (TDO) . N-formylkynurenine is degraded by formamidase to yield the pivotal metabolite kynurenine. Compared to peripheral organs, the activities of these enzymes in the brain are normally low. Kynurenine undergoes irreversible transamination to form kynurenic acid (KYNA). Four kynurenine aminotransferases (KATs) have so far been shown to catalyze this reaction . In the mammalian brain, KAT II is thought to be the main biosynthetic enzyme of KYNA as it does not recognize competing abundant amino acids as substrates . No degradative enzyme of KYNA has yet been described in the mammalian brain. A second branch of the kynurenine pathway, competing with KYNA synthesis by KATs, is initiated by kynurenine 3-monooxygenase (KMO) and kynureninase, which catalyze the degradation of kynurenine to 3-hydroxykynurenine (3-HK) and anthranilic acid, respectively . The in vivo disposition of kynurenine in the brain depends on the cellular localization, intracellular compartmentalization and kinetic characteristics of these two degradative enzymes, as well as KATs , . Like kynurenine, 3-HK serves as a substrate of competing enzymes: kynureninase and KAT II. Mammalian kynureninase preferentially recognizes 3-HK over kynurenine, catalyzing the formation of 3-HANA . Compared to peripheral organs and for unknown reasons, anthranilic acid is a far better precursor of 3-HANA than 3-HK within the brain . KAT II, and possibly other KATs, convert 3-HK to xanthurenic acid, which, like KYNA, appears to constitute a dead-end product of the kynurenine pathway. In addition to being oxidized non-enzymatically to cinnabarinic acid, 3-HANA is the substrate of 3-hydroxyanthranilic acid 3,4-dioxygenase (3HAO), which is present in relative abundance in the brain , forming α-amino-α-carboxymuconic-ω-semialdehyde. The kynurenine pathway branches then branches into two arms. Under physiological conditions, α-amino-α-carboxymuconic-ω-semialdehyde spontaneously rearranges to form QUIN with a half-life of approximately 20 minutes. QUIN synthesis may therefore be regulated both at the level of 3HAO (for example by Fe2+) and by changes in the intracellular milieu (pH, temperature) to influence the second, non-enzymatic step. Notably, the brain seems to contain very little α-amino-α-carboxymuconic-ω-semialdehyde carboxylase, an enzyme which deflects the metabolic cascade towards the production of picolinic and glutaric acids . The cerebral activity of QUIN's degradative enzyme, quinolinate phosphoribosyltransferase, is very low , making this enzyme one of the gatekeepers for the synthesis of NAD+ –.
Figure 2. Segregation of the two Kynurenine Pathway Branches in the Brain
Under physiological conditions, kynurenine pathway enzymes in the mammalian brain are preferentially, although not exclusively, localized in non-neuronal cells –. Metabolism of the pathway is driven by blood-derived tryptophan (TRP), kynurenine (KYN) or 3-hydroxykynurenine (3-HK), or by locally formed metabolites. Of functional significance, the two branches of the pathway are physically segregated in the brain. Astrocytes, which harbor KATs but do not contain KMO and therefore cannot produce 3-HK from KYN , –, account for kynurenic acid (KYNA) biosynthesis, which is regulated by intracellular metabolic events –. 3-HK and its major downstream metabolites are synthesized in microglial and other cells of monocytic origin , . Once synthesized within glial cells, quinolinic acid (QUIN) and KYNA are promptly released into the extracellular milieu to affect their pre- and postsynaptic (“pre” and “post”) neuronal targets. 3-HANA: 3-Hydroxyanthranilic acid, α7nAChR: α7 nicotinic acetylcholine receptor, TCA: Tricarboxylic acid.
Figure 3. Communication between Peripheral and Central Kynurenine Pathways under Normal and Inflammatory Conditions
A: In the periphery, the degradation of tryptophan and the subsequent formation of circulating kynurenines is normally regulated by steroid hormones, cytokines and growth factors. These factors stimulate IDO , , and also activate, or lead to the up-regulation of, other kynurenine pathway enzymes including TDO and KMO , . Jointly, they control the levels of circulating tryptophan, kynurenine and 3-HK, which all readily cross the blood-brain barrier using the large neutral amino acid transporter . Together with kynurenine pathway metabolism within glial cells, brain uptake of these kynurenines therefore determines kynurenine pathway flux in the brain; B: Inflammatory conditions stimulate the kynurenine pathway both in the periphery and in the brain. Activation of kynurenine pathway enzymes, especially IDO and KMO, in peripheral immune cells – such as dendritic cells and macrophages – promotes the production of kynurenine and its downstream metabolites in the blood. Increased influx of the brain-permeable metabolites, in some cases aided by a leaky blood-brain barrier , as well as infiltration of macrophages or microbial pathogens , then leads to an excess of kynurenines in the brain parenchyma. Furthermore, infiltrating cytokines stimulate the kynurenine pathway in activated microglial cells and blood-borne cells within the brain . Together, these peripheral and central events connect neuroinflammatory mechanisms with abnormal metabolism along the kynurenine pathway, and brain pathology.
Figure 3. Communication between Peripheral and Central Kynurenine Pathways under Normal and Inflammatory Conditions
A: In the periphery, the degradation of tryptophan and the subsequent formation of circulating kynurenines is normally regulated by steroid hormones, cytokines and growth factors. These factors stimulate IDO , , and also activate, or lead to the up-regulation of, other kynurenine pathway enzymes including TDO and KMO , . Jointly, they control the levels of circulating tryptophan, kynurenine and 3-HK, which all readily cross the blood-brain barrier using the large neutral amino acid transporter . Together with kynurenine pathway metabolism within glial cells, brain uptake of these kynurenines therefore determines kynurenine pathway flux in the brain; B: Inflammatory conditions stimulate the kynurenine pathway both in the periphery and in the brain. Activation of kynurenine pathway enzymes, especially IDO and KMO, in peripheral immune cells – such as dendritic cells and macrophages – promotes the production of kynurenine and its downstream metabolites in the blood. Increased influx of the brain-permeable metabolites, in some cases aided by a leaky blood-brain barrier , as well as infiltration of macrophages or microbial pathogens , then leads to an excess of kynurenines in the brain parenchyma. Furthermore, infiltrating cytokines stimulate the kynurenine pathway in activated microglial cells and blood-borne cells within the brain . Together, these peripheral and central events connect neuroinflammatory mechanisms with abnormal metabolism along the kynurenine pathway, and brain pathology.
Figure 4. Effects of Kynurenine Pathway Manipulation on Cognition and Neurodegeneration
A: Inhibition of endogenous KYNA synthesis with the KAT II inhibitor (S)-4-(ethylsulfonyl)benzoylalanine (ESBA), administered intracerebroventricularly 90 min prior to daily training (Days 1–4), facilitates learning in a spatial working memory task (the Morris water maze test) in normal rats. Co-administration of KYNA eliminates this effect (see for details); B: JM6, a peripherally acting pro-drug of the KMO inhibitor Ro 61-8048, prevents synaptic loss in a transgenic mouse model of Alzheimer’s disease (APPtg mice). JM6 was administered orally for 120 days, and the brain was evaluated in 8-month-old APPtg mice. Left: Representative image (630X) of serial sections of the hippocampus of wild-type or APPtg mice immunostained with an antibody for synaptophysin. Right: Quantification of synaptophysin levels in the hippocampus of APPtg mice treated with JM6. Synaptophysin levels in APPtg mice treated with JM6 are not significantly different from those found in wild-type mice (see for details).
Figure 4. Effects of Kynurenine Pathway Manipulation on Cognition and Neurodegeneration
A: Inhibition of endogenous KYNA synthesis with the KAT II inhibitor (S)-4-(ethylsulfonyl)benzoylalanine (ESBA), administered intracerebroventricularly 90 min prior to daily training (Days 1–4), facilitates learning in a spatial working memory task (the Morris water maze test) in normal rats. Co-administration of KYNA eliminates this effect (see for details); B: JM6, a peripherally acting pro-drug of the KMO inhibitor Ro 61-8048, prevents synaptic loss in a transgenic mouse model of Alzheimer’s disease (APPtg mice). JM6 was administered orally for 120 days, and the brain was evaluated in 8-month-old APPtg mice. Left: Representative image (630X) of serial sections of the hippocampus of wild-type or APPtg mice immunostained with an antibody for synaptophysin. Right: Quantification of synaptophysin levels in the hippocampus of APPtg mice treated with JM6. Synaptophysin levels in APPtg mice treated with JM6 are not significantly different from those found in wild-type mice (see for details).
Figure 5. Targeting Brain Kynurenines Pharmacologically: Focus on KAT II and KMO
As KAT II and KMO are directly responsible for the formation of KYNA and 3-HK, respectively, specific inhibitors of these enzymes can be used to manipulate the ratio between these two neuroactive metabolites pharmacologically. Increases in the KYNA:3-HK ratio are desirable in neurodegenerative diseases such as Huntington’s disease, which present with an excess of neurotoxic 3-HK (and QUIN) in the brain , , . Conversely, lowering of the KYNA:3-HK ratio in the brain will improve cognitive capabilities, providing special benefits in diseases such as schizophrenia, which show enhanced brain levels of KYNA . In the normal brain, fluctuations in the KYNA:3-HK ratio will have moderate effects on neuronal viability and cognition, limited to the physiological range. Pharmacological interventions with specific “kynurenergic” agents, such as KAT II or KMO inhibitors, should therefore be tailored to achieve the intended benefits and minimize potentially harmful consequences. The gray areas indicate transition zones between the physiological range and pathologies.
Similar articles
- The kynurenine pathway and the brain: Challenges, controversies and promises.
Schwarcz R, Stone TW. Schwarcz R, et al. Neuropharmacology. 2017 Jan;112(Pt B):237-247. doi: 10.1016/j.neuropharm.2016.08.003. Epub 2016 Aug 7. Neuropharmacology. 2017. PMID: 27511838 Free PMC article. Review. - Brain Aging and Disorders of the Central Nervous System: Kynurenines and Drug Metabolism.
Török N, Majláth Z, Fülöp F, Toldi J, Vécsei L. Török N, et al. Curr Drug Metab. 2016;17(5):412-29. doi: 10.2174/1389200217666151222155043. Curr Drug Metab. 2016. PMID: 26694727 Review. - Kynurenine metabolites of tryptophan: implications for neurologic diseases.
Freese A, Swartz KJ, During MJ, Martin JB. Freese A, et al. Neurology. 1990 Apr;40(4):691-5. doi: 10.1212/wnl.40.4.691. Neurology. 1990. PMID: 2181342 Review. - Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases.
Lovelace MD, Varney B, Sundaram G, Lennon MJ, Lim CK, Jacobs K, Guillemin GJ, Brew BJ. Lovelace MD, et al. Neuropharmacology. 2017 Jan;112(Pt B):373-388. doi: 10.1016/j.neuropharm.2016.03.024. Epub 2016 Mar 16. Neuropharmacology. 2017. PMID: 26995730 Review. - Effects of immune activation on quinolinic acid and neuroactive kynurenines in the mouse.
Saito K, Markey SP, Heyes MP. Saito K, et al. Neuroscience. 1992 Nov;51(1):25-39. doi: 10.1016/0306-4522(92)90467-g. Neuroscience. 1992. PMID: 1465184
Cited by
- Unveiling tryptophan dynamics and functions across model organisms via quantitative imaging.
Wang K, Chen TL, Zhang XX, Cao JB, Wang P, Wang M, Du JL, Mu Y, Tao R. Wang K, et al. BMC Biol. 2024 Nov 14;22(1):258. doi: 10.1186/s12915-024-02058-x. BMC Biol. 2024. PMID: 39538250 Free PMC article. - Baseline Inflammation but not Exercise Modality Impacts Exercise-induced Kynurenine Pathway Modulation in Persons With Multiple Sclerosis: Secondary Results From a Randomized Controlled Trial.
Kupjetz M, Patt N, Joisten N, Ueland PM, McCann A, Gonzenbach R, Bansi J, Zimmer P. Kupjetz M, et al. Int J Tryptophan Res. 2024 Nov 11;17:11786469241284423. doi: 10.1177/11786469241284423. eCollection 2024. Int J Tryptophan Res. 2024. PMID: 39534856 Free PMC article. - No Effects of Omega-3 Supplementation on Kynurenine Pathway, Inflammation, Depressive Symptoms, and Stress Response in Males: A Placebo-Controlled Trial.
Bidzan-Wiącek M, Tomczyk M, Błażek M, Mika A, Antosiewicz J. Bidzan-Wiącek M, et al. Nutrients. 2024 Oct 31;16(21):3744. doi: 10.3390/nu16213744. Nutrients. 2024. PMID: 39519577 Free PMC article. Clinical Trial. - Gut-brain axis and neurodegeneration: mechanisms and therapeutic potentials.
Park KJ, Gao Y. Park KJ, et al. Front Neurosci. 2024 Oct 23;18:1481390. doi: 10.3389/fnins.2024.1481390. eCollection 2024. Front Neurosci. 2024. PMID: 39513042 Free PMC article. Review. - Tryptophan Metabolism Disorder-Triggered Diseases, Mechanisms, and Therapeutic Strategies: A Scientometric Review.
Chen X, Xu D, Yu J, Song XJ, Li X, Cui YL. Chen X, et al. Nutrients. 2024 Oct 4;16(19):3380. doi: 10.3390/nu16193380. Nutrients. 2024. PMID: 39408347 Free PMC article. Review.
References
- Liebig J. Über Kynurensäure. Justus Liebig's Ann. Chem. 1853;86:125–126.
- Leklem JE. Quantitative aspects of tryptophan metabolism in humans and other species: a review. Am. J. Clin. Nutr. 1971;24:659–672. - PubMed
- Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247:184–187. - PubMed
- Parsons CG, et al. Novel systemically active antagonists of the glycine site of the N-methyl-D-aspartate receptor: electrophysiological, biochemical and behavioral characterization. J. Pharmacol. Exp. Ther. 1997;283:1264–1275. - PubMed
- Maj J, Rogoz Z, Skuza G, Kolodziejczyk K. Some central effects of kynurenic acid, 7-chlorokynurenic acid and 5,7-dichloro-kynurenic acid, glycine site antagonists. Pol. J. Pharmacol. 1994;46:115–124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical