Research resource: whole transcriptome RNA sequencing detects multiple 1α,25-dihydroxyvitamin D(3)-sensitive metabolic pathways in developing zebrafish - PubMed (original) (raw)
Research resource: whole transcriptome RNA sequencing detects multiple 1α,25-dihydroxyvitamin D(3)-sensitive metabolic pathways in developing zebrafish
Theodore A Craig et al. Mol Endocrinol. 2012 Sep.
Abstract
The biological role of vitamin D receptors (VDR), which are abundantly expressed in developing zebrafish (Danio rerio) as early as 48 h after fertilization, and before the development of a mineralized skeleton and mature intestine and kidney, is unknown. We probed the role of VDR in developing zebrafish biology by examining changes in expression of RNA by whole transcriptome shotgun sequencing (RNA-seq) in fish treated with picomolar concentrations of the VDR ligand and hormonal form of vitamin D(3), 1α,25-dihydroxyvitamin D(3) [1α,25(OH)(2)D(3))].We observed significant changes in RNAs of transcription factors, leptin, peptide hormones, and RNAs encoding proteins of fatty acid, amino acid, xenobiotic metabolism, receptor-activator of NFκB ligand (RANKL), and calcitonin-like ligand receptor pathways. Early highly restricted, and subsequent massive changes in more than 10% of expressed cellular RNA were observed. At days post fertilization (dpf) 2 [24 h 1α,25(OH)(2)D(3)-treatment], only four RNAs were differentially expressed (hormone vs. vehicle). On dpf 4 (72 h treatment), 77 RNAs; on dpf 6 (120 h treatment) 1039 RNAs; and on dpf 7 (144 h treatment), 2407 RNAs were differentially expressed in response to 1α,25(OH)(2)D(3). Fewer RNAs (n = 481) were altered in dpf 7 larvae treated for 24 h with 1α,25(OH)(2)D(3) vs. those treated with hormone for 144 h. At dpf 7, in 1α,25(OH)(2)D(3)-treated larvae, pharyngeal cartilage was larger and mineralization was greater. Changes in expression of RNAs for transcription factors, peptide hormones, and RNAs encoding proteins integral to fatty acid, amino acid, leptin, calcitonin-like ligand receptor, RANKL, and xenobiotic metabolism pathways, demonstrate heretofore unrecognized mechanisms by which 1α,25(OH)(2)D(3) functions in vivo in developing eukaryotes.
Figures
Fig. 1.
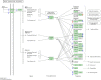
Experimental design. Experiment 1: zebrafish embryos were treated with 1α,25(OH)2D3 (300 p
m
) or vehicle beginning at 24 hpf. Medium and additives were changed to every 24 h (black arrows). Five batches of 25–30, 1α,25(OH)2D3-treated, and five batches of 25–30 vehicle-treated zebrafish embryos/larvae were collected at 2, 4, 6, and 7 dpf (red arrows) for isolation of RNA, construction of cDNA libraries, and sequencing. Experiment 2: zebrafish larvae were treated with 1α,25(OH)2D3 (300 p
m
) or vehicle beginning at 144 hpf; 24 h later, three batches of 25–30 1α,25(OH)2D3-treated and three batches of 25–30 vehicle-treated zebrafish were collected (red arrow) for isolation of RNA, construction of cDNA libraries, and sequencing.
Fig. 2.
PPAR signaling pathway is significantly altered by 1α,25(OH)2D3 treatment on dpf 6 [120-h 1α,25(OH)2D3 treatment]. RNAs increased after 1α,25(OH)2D3 treatment (compared with vehicle) are indicated by red up arrows, and RNAs decreased after 1α,25(OH)2D3 treatment are indicated by blue down arrows. RXR, Retinoid X receptor; UCP, uncoupling protein; VLDL, very-low density lipoprotein.
Fig. 3.
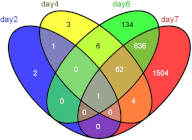
Venn diagram showing the developmental distribution of the genes altered by 1α,25(OH)2D3 treatment. Genes were grouped based on their altered expression at single or multiple stages. Differential expression analyses were evaluated between ethanol and 1α,25(OH)2D3 treatments on dpf 2, 4, 6, and 7. In total, 2553 mRNA transcripts were differentially expressed in at least one the four studied developmental stages.
Fig. 4.
Alcian blue-stained, dpf 7 (168 hpf) zebrafish larvae treated with ethanol (vehicle, EtOH) or 1α,25(OH)2D3 (300 p
m
) continuously for 6 d (144 h). The length of the palatoquadrate (PQ) and ceratohyal (CH) cartilages was assessed in each embryo. The palatoquadrate and ceratohyal cartilages were larger in 1α,25(OH)2D3-treated zebrafish compared with vehicle-treated zebrafish (palatoquadrate P = 0.0005; ceratohyal P = 0.015). A1, B1, and C1 represent ethanol-treated zebrafish, lateral view; A2, B2, and C2 represent ethanol-treated zebrafish, ventral view. D1, E1, and F1 represent 1α,25(OH)2D3-treated, lateral view; D2, E2, and F2 represent 1α,25(OH)2D3-treated, ventral view. VitD, Vitamin D.
Fig. 5.
Alizarin red-stained, dpf 7 (168 hpf) zebrafish larvae treated with ethanol (vehicle, EtOH) or 1α,25(OH)2D3 (300 p
m
) continuously for 6 d (144 h). The mineralized skeleton appears darker in the 1α,25(OH)2D3-treated zebrafish. n, Notochord. A1, B1, and C1 represent ethanol-treated zebrafish, lateral view; A2, B2, C2 represent ethanol-treated zebrafish, ventral view. D1, E1, and F1 represent 1α,25(OH)2D3-treated, lateral view; D2, E2, and F2 represent 1α,25(OH)2D3-treated, ventral view. VitD, Vitamin D.
Similar articles
- Detection of 1α,25-dihydroxyvitamin D-regulated miRNAs in zebrafish by whole transcriptome sequencing.
Craig TA, Zhang Y, Magis AT, Funk CC, Price ND, Ekker SC, Kumar R. Craig TA, et al. Zebrafish. 2014 Jun;11(3):207-18. doi: 10.1089/zeb.2013.0899. Epub 2014 Mar 20. Zebrafish. 2014. PMID: 24650217 Free PMC article. - Expression and regulation of the vitamin D receptor in the zebrafish, Danio rerio.
Craig TA, Sommer S, Sussman CR, Grande JP, Kumar R. Craig TA, et al. J Bone Miner Res. 2008 Sep;23(9):1486-96. doi: 10.1359/jbmr.080403. J Bone Miner Res. 2008. PMID: 18410235 Free PMC article. - Dynamics of 1α,25-dihydroxyvitamin D3-dependent chromatin accessibility of early vitamin D receptor target genes.
Seuter S, Pehkonen P, Heikkinen S, Carlberg C. Seuter S, et al. Biochim Biophys Acta. 2013 Dec;1829(12):1266-75. doi: 10.1016/j.bbagrm.2013.10.003. Epub 2013 Nov 1. Biochim Biophys Acta. 2013. PMID: 24185200 - The vitamin D hormone and its nuclear receptor: molecular actions and disease states.
Haussler MR, Haussler CA, Jurutka PW, Thompson PD, Hsieh JC, Remus LS, Selznick SH, Whitfield GK. Haussler MR, et al. J Endocrinol. 1997 Sep;154 Suppl:S57-73. J Endocrinol. 1997. PMID: 9379138 Review. - Update on biological actions of 1alpha,25(OH)2-vitamin D3 (rapid effects) and 24R,25(OH)2-vitamin D3.
Norman AW, Okamura WH, Bishop JE, Henry HL. Norman AW, et al. Mol Cell Endocrinol. 2002 Nov 29;197(1-2):1-13. doi: 10.1016/s0303-7207(02)00273-3. Mol Cell Endocrinol. 2002. PMID: 12431790 Review.
Cited by
- Enhanced insulin activity achieved in VDRa/b ablation zebrafish.
Liu R, Lu Y, Peng X, Jia J, Ruan Y, Shi S, Shu T, Li T, Jin X, Zhai G, He J, Lou Q, Yin Z. Liu R, et al. Front Endocrinol (Lausanne). 2023 Feb 13;14:1054665. doi: 10.3389/fendo.2023.1054665. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36864841 Free PMC article. - Comparative analysis of nutritional guidelines for vitamin D.
Bouillon R. Bouillon R. Nat Rev Endocrinol. 2017 Aug;13(8):466-479. doi: 10.1038/nrendo.2017.31. Epub 2017 Apr 7. Nat Rev Endocrinol. 2017. PMID: 28387318 Review. - Methylarginine metabolites are associated with attenuated muscle protein synthesis in cancer-associated muscle wasting.
Kunz HE, Dorschner JM, Berent TE, Meyer T, Wang X, Jatoi A, Kumar R, Lanza IR. Kunz HE, et al. J Biol Chem. 2020 Dec 18;295(51):17441-17459. doi: 10.1074/jbc.RA120.014884. J Biol Chem. 2020. PMID: 33453990 Free PMC article. - Short stories on zebrafish long noncoding RNAs.
Haque S, Kaushik K, Leonard VE, Kapoor S, Sivadas A, Joshi A, Scaria V, Sivasubbu S. Haque S, et al. Zebrafish. 2014 Dec;11(6):499-508. doi: 10.1089/zeb.2014.0994. Zebrafish. 2014. PMID: 25110965 Free PMC article. Review. - Zebrafish Bone and General Physiology Are Differently Affected by Hormones or Changes in Gravity.
Aceto J, Nourizadeh-Lillabadi R, Marée R, Dardenne N, Jeanray N, Wehenkel L, Aleström P, van Loon JJ, Muller M. Aceto J, et al. PLoS One. 2015 Jun 10;10(6):e0126928. doi: 10.1371/journal.pone.0126928. eCollection 2015. PLoS One. 2015. PMID: 26061167 Free PMC article.
References
- DeLuca HF. 2004. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 80:1689S–1696S - PubMed
- Kumar R. 1991. Vitamin D and calcium transport. Kidney Int 40:1177–1189 - PubMed
- Haussler MR, Haussler CA, Bartik L, Whitfield GK, Hsieh JC, Slater S, Jurutka PW. 2008. Vitamin D receptor: molecular signaling and actions of nutritional ligands in disease prevention. Nutr Rev 66:S98–S112 - PubMed
- Wasserman RH, Smith CA, Brindak ME, De Talamoni N, Fullmer CS, Penniston JT, Kumar R. 1992. Vitamin D and mineral deficiencies increase the plasma membrane calcium pump of chicken intestine. Gastroenterology 102:886–894 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR-058003/AR/NIAMS NIH HHS/United States
- AR-60869/AR/NIAMS NIH HHS/United States
- 4R00CA126184/CA/NCI NIH HHS/United States
- R21 AR060869/AR/NIAMS NIH HHS/United States
- R00 CA126184/CA/NCI NIH HHS/United States
- R21 AR058003/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases