Membrane vesicle formation as a multiple-stress response mechanism enhances Pseudomonas putida DOT-T1E cell surface hydrophobicity and biofilm formation - PubMed (original) (raw)
Membrane vesicle formation as a multiple-stress response mechanism enhances Pseudomonas putida DOT-T1E cell surface hydrophobicity and biofilm formation
Thomas Baumgarten et al. Appl Environ Microbiol. 2012 Sep.
Abstract
Among the adaptive responses of bacteria to rapid changes in environmental conditions, those of the cell envelope are known to be the most crucial. Therefore, several mechanisms with which bacteria change their cell surface and membranes in the presence of different environmental stresses have been elucidated. Among these mechanisms, the release of outer membrane vesicles (MV) in Gram-negative bacteria has attracted particular research interest because of its involvement in pathogenic processes, such as that of Pseudomonas aeruginosa biofilm formation in cystic fibrosis lungs. In this study, we investigated the role of MV formation as an adaptive response of Pseudomonas putida DOT-T1E to several environmental stress factors and correlated it to the formation of biofilms. In the presence of toxic concentrations of long-chain alcohols, under osmotic stress caused by NaCl, in the presence of EDTA, and after heat shock, cells of this strain released MV within 10 min in the presence of a stressor. The MV formed showed similar size and charge properties, as well as comparable compositions of proteins and fatty acids. MV release caused a significant increase in cell surface hydrophobicity, and an enhanced tendency to form biofilms was demonstrated in this study. Therefore, the release of MV as a stress response could be put in a physiological context.
Figures
Fig 1
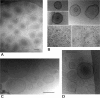
Representative cryo-transmission electron microscopy images of MV formed by P. putida DOT-T1E after incubation with 1-octanol (A), NaCl (B), heat shock (C), and EDTA (D). Bars, 200 nm.
Fig 2
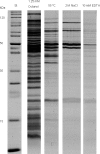
Comparative overview of SDS-PAGE results of the isolated MV. Lane 1, standard; lane 2, 1-octanol-induced MV; lane 3, heat shock-induced MV; lane 4, NaCl-induced MV; lane 5, EDTA-induced MV.
Fig 3
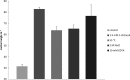
Effects of 1-octanol (A), heat shock (B), NaCl (C), and EDTA (D) on water contact angles of growing cells of P. putida DOT-T1E. Error bars show standard deviations.
Fig 4
Microscopic images of biofilms formed by P. putida on polystyrene microtiter wells after incubation with HgCl2 (A), 1-octanol (B), heat shock (C), NaCl (D), and EDTA (E).
Fig 5
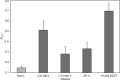
Quantification of biofilm formation of P. putida after incubation with different stressors. Error bars show standard deviations.
Fig 6
Possible sequence of biofilm formation in P. putida after exposure to a stressor.
Similar articles
- Alkanols and chlorophenols cause different physiological adaptive responses on the level of cell surface properties and membrane vesicle formation in Pseudomonas putida DOT-T1E.
Baumgarten T, Vazquez J, Bastisch C, Veron W, Feuilloley MG, Nietzsche S, Wick LY, Heipieper HJ. Baumgarten T, et al. Appl Microbiol Biotechnol. 2012 Jan;93(2):837-45. doi: 10.1007/s00253-011-3442-9. Epub 2011 Jul 6. Appl Microbiol Biotechnol. 2012. PMID: 21732242 - Responses of unsaturated Pseudomonas putida CZ1 biofilms to environmental stresses in relation to the EPS composition and surface morphology.
Lin H, Chen G, Long D, Chen X. Lin H, et al. World J Microbiol Biotechnol. 2014 Dec;30(12):3081-90. doi: 10.1007/s11274-014-1735-8. Epub 2014 Sep 13. World J Microbiol Biotechnol. 2014. PMID: 25217027 - Quantification of outer membrane vesicles: a potential tool to compare response in Pseudomonas putida KT2440 to stress caused by alkanols.
Eberlein C, Starke S, Doncel ÁE, Scarabotti F, Heipieper HJ. Eberlein C, et al. Appl Microbiol Biotechnol. 2019 May;103(10):4193-4201. doi: 10.1007/s00253-019-09812-0. Epub 2019 Apr 10. Appl Microbiol Biotechnol. 2019. PMID: 30972462 - Immediate response mechanisms of Gram-negative solvent-tolerant bacteria to cope with environmental stress: cis-trans isomerization of unsaturated fatty acids and outer membrane vesicle secretion.
Eberlein C, Baumgarten T, Starke S, Heipieper HJ. Eberlein C, et al. Appl Microbiol Biotechnol. 2018 Mar;102(6):2583-2593. doi: 10.1007/s00253-018-8832-9. Epub 2018 Feb 15. Appl Microbiol Biotechnol. 2018. PMID: 29450619 Free PMC article. Review. - Dynamics of development and dispersal in sessile microbial communities: examples from Pseudomonas aeruginosa and Pseudomonas putida model biofilms.
Klausen M, Gjermansen M, Kreft JU, Tolker-Nielsen T. Klausen M, et al. FEMS Microbiol Lett. 2006 Aug;261(1):1-11. doi: 10.1111/j.1574-6968.2006.00280.x. FEMS Microbiol Lett. 2006. PMID: 16842351 Review.
Cited by
- Functional advantages conferred by extracellular prokaryotic membrane vesicles.
Manning AJ, Kuehn MJ. Manning AJ, et al. J Mol Microbiol Biotechnol. 2013;23(1-2):131-41. doi: 10.1159/000346548. Epub 2013 Apr 18. J Mol Microbiol Biotechnol. 2013. PMID: 23615201 Free PMC article. Review. - Outer membrane vesicles and the outer membrane protein OmpU govern Vibrio cholerae biofilm matrix assembly.
Potapova A, Garvey W, Dahl P, Guo S, Chang Y, Schwechheimer C, Trebino MA, Floyd KA, Phinney BS, Liu J, Malvankar NS, Yildiz FH. Potapova A, et al. mBio. 2024 Feb 14;15(2):e0330423. doi: 10.1128/mbio.03304-23. Epub 2024 Jan 11. mBio. 2024. PMID: 38206049 Free PMC article. - Membrane Vesicles of Group B Streptococcus Disrupt Feto-Maternal Barrier Leading to Preterm Birth.
Surve MV, Anil A, Kamath KG, Bhutda S, Sthanam LK, Pradhan A, Srivastava R, Basu B, Dutta S, Sen S, Modi D, Banerjee A. Surve MV, et al. PLoS Pathog. 2016 Sep 1;12(9):e1005816. doi: 10.1371/journal.ppat.1005816. eCollection 2016 Sep. PLoS Pathog. 2016. PMID: 27583406 Free PMC article. - EPS Glycoconjugate Profiles Shift as Adaptive Response in Anaerobic Microbial Granulation at High Salinity.
Gagliano MC, Neu TR, Kuhlicke U, Sudmalis D, Temmink H, Plugge CM. Gagliano MC, et al. Front Microbiol. 2018 Jul 2;9:1423. doi: 10.3389/fmicb.2018.01423. eCollection 2018. Front Microbiol. 2018. PMID: 30013532 Free PMC article. - Bacterial lipopolysaccharide induces settlement and metamorphosis in a marine larva.
Freckelton ML, Nedved BT, Cai YS, Cao S, Turano H, Alegado RA, Hadfield MG. Freckelton ML, et al. Proc Natl Acad Sci U S A. 2022 May 3;119(18):e2200795119. doi: 10.1073/pnas.2200795119. Epub 2022 Apr 25. Proc Natl Acad Sci U S A. 2022. PMID: 35467986 Free PMC article.
References
- Bastida F, et al. 2010. Elucidating MTBE degradation in a mixed consortium using a multidisciplinary approach. FEMS Microbiol. Ecol. 73: 370–384 - PubMed
- Baumgarten T, et al. 2012. Alkanols and chlorophenols cause different physiological adaptive responses on the level of cell surface properties and membrane vesicle formation in Pseudomonas putida DOT-T1E. Appl. Microbiol. Biotechnol. 93: 837–845 - PubMed
- Beveridge TJ, Makin SA, Kadurugamuwa JL, Li ZS. 1997. Interactions between biofilms and the environment. FEMS Microbiol. Rev. 20: 291–303 - PubMed
- Choi DS, et al. 2011. Proteomic analysis of outer membrane vesicles derived from Pseudomonas aeruginosa . Proteomics 11: 3424–3429 - PubMed
- de Carvalho C, Wick LY, Heipieper HJ. 2009. Cell wall adaptations of planktonic and biofilm Rhodococcus erythropolis cells to growth on C5 to C16 n-alkane hydrocarbons. Appl. Microbiol. Biotechnol. 82: 311–320 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources