Impact of postprandial glycaemia on health and prevention of disease - PubMed (original) (raw)
Review
. 2012 Oct;13(10):923-84.
doi: 10.1111/j.1467-789X.2012.01011.x. Epub 2012 Jul 11.
J-M Antoine, D Benton, I Björck, L Bozzetto, F Brouns, M Diamant, L Dye, T Hulshof, J J Holst, D J Lamport, M Laville, C L Lawton, A Meheust, A Nilson, S Normand, A A Rivellese, S Theis, S S Torekov, S Vinoy
Affiliations
- PMID: 22780564
- PMCID: PMC3494382
- DOI: 10.1111/j.1467-789X.2012.01011.x
Free PMC article
Review
Impact of postprandial glycaemia on health and prevention of disease
E E Blaak et al. Obes Rev. 2012 Oct.
Free PMC article
Abstract
Postprandial glucose, together with related hyperinsulinemia and lipidaemia, has been implicated in the development of chronic metabolic diseases like obesity, type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD). In this review, available evidence is discussed on postprandial glucose in relation to body weight control, the development of oxidative stress, T2DM, and CVD and in maintaining optimal exercise and cognitive performance. There is mechanistic evidence linking postprandial glycaemia or glycaemic variability to the development of these conditions or in the impairment in cognitive and exercise performance. Nevertheless, postprandial glycaemia is interrelated with many other (risk) factors as well as to fasting glucose. In many studies, meal-related glycaemic response is not sufficiently characterized, or the methodology with respect to the description of food or meal composition, or the duration of the measurement of postprandial glycaemia is limited. It is evident that more randomized controlled dietary intervention trials using effective low vs. high glucose response diets are necessary in order to draw more definite conclusions on the role of postprandial glycaemia in relation to health and disease. Also of importance is the evaluation of the potential role of the time course of postprandial glycaemia.
© 2012 ILSI Europe. obesity reviews © 2012 International Association for the Study of Obesity.
Figures
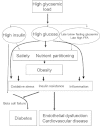
Figure 1
Putative relationships between postprandial glycaemia and risk factors for obesity, diabetes and cardiovascular disease. FFA, free fatty acid.
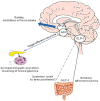
Figure 2
Glucagon-like peptide-1 (GLP-1) increase insulin secretion lowers blood glucose and inhibits appetite. The ingestion of food promotes the release of GLP-1 from L-cells in the intestine, which activates vagal afferents. Activated GLP-1 neurons of the nucleus of the solitary tract (NTS) project to the hypothalamic arcuate nucleus (ARC), to modulate vagal motor outflow to the pancreas and other tissues not depicted, increasing insulin secretion from the β-cells in states of hyperglycaemia and suppresses glucagon from the α-cells, leading to lowering of blood glucose. Systemic GLP-1 may also access the brain via leaks in the blood–brain barrier such as the subfornical organ and the area postrema, as demonstrated to occur in rats. In the brain, release of GLP-1 within the NTS and from projections of GLP-1 neurons to the paraventricular neurons (PVN) leads to GLP-1-R activation, which promotes satiety and anorexia. Besides from the actions depicted in the picture GLP-1 has numerous other actions including slowing gastric emptying and thereby flattening glucose excursions, as well as cardiac effects. Adapted from Torekov et al., Obesity Reviews 2011 , and Williams, Endocrinology. 2009; 150: 2997–3001 .
Similar articles
- Summation of blood glucose and TAG to characterise the 'metabolic load index'.
Emerson SR, Haub MD, Teeman CS, Kurti SP, Rosenkranz SK. Emerson SR, et al. Br J Nutr. 2016 Nov;116(9):1553-1563. doi: 10.1017/S0007114516003585. Epub 2016 Oct 24. Br J Nutr. 2016. PMID: 27774915 Review. - Postprandial dysmetabolism and large-vessel disease.
Segal P. Segal P. Diabetes Nutr Metab. 2002 Dec;15(6 Suppl):3-8. Diabetes Nutr Metab. 2002. PMID: 12702001 Review. - HbA₁(c) and mean blood glucose show stronger associations with cardiovascular disease risk factors than do postprandial glycaemia or glucose variability in persons with diabetes: the A1C-Derived Average Glucose (ADAG) study.
Borg R, Kuenen JC, Carstensen B, Zheng H, Nathan DM, Heine RJ, Nerup J, Borch-Johnsen K, Witte DR; ADAG Study Group. Borg R, et al. Diabetologia. 2011 Jan;54(1):69-72. doi: 10.1007/s00125-010-1918-2. Epub 2010 Oct 1. Diabetologia. 2011. PMID: 20886203 Free PMC article. - Glycaemic glucose equivalent: combining carbohydrate content, quantity and glycaemic index of foods for precision in glycaemia management.
Monro JA. Monro JA. Asia Pac J Clin Nutr. 2002;11(3):217-25. doi: 10.1046/j.1440-6047.2002.00295.x. Asia Pac J Clin Nutr. 2002. PMID: 12230236 - Postprandial glycaemia: a plea for the frequent use of delta postprandial glycaemia in the treatment of diabetic patients.
Slama G, Elgrably F, Sola A, Mbemba J, Larger E. Slama G, et al. Diabetes Metab. 2006 Apr;32(2):187-92. doi: 10.1016/s1262-3636(07)70268-9. Diabetes Metab. 2006. PMID: 16735970
Cited by
- Polyphenol-rich diets improve glucose metabolism in people at high cardiometabolic risk: a controlled randomised intervention trial.
Bozzetto L, Annuzzi G, Pacini G, Costabile G, Vetrani C, Vitale M, Griffo E, Giacco A, De Natale C, Cocozza S, Della Pepa G, Tura A, Riccardi G, Rivellese AA. Bozzetto L, et al. Diabetologia. 2015 Jul;58(7):1551-60. doi: 10.1007/s00125-015-3592-x. Epub 2015 Apr 24. Diabetologia. 2015. PMID: 25906754 Clinical Trial. - Ultra-processed foods intake in relation to metabolic health status, serum brain-derived neurotrophic factor and adropin levels in adults.
Poursalehi D, Tirani SA, Shahdadian F, Hajhashemy Z, Rouhani P, Saneei P. Poursalehi D, et al. Nutr J. 2024 Oct 9;23(1):121. doi: 10.1186/s12937-024-01024-1. Nutr J. 2024. PMID: 39385201 Free PMC article. - Testing the Utility of Polygenic Risk Scores for Type 2 Diabetes and Obesity in Predicting Metabolic Changes in a Prediabetic Population: An Observational Study.
Padilla-Martinez F, Szczerbiński Ł, Citko A, Czajkowski M, Konopka P, Paszko A, Wawrusiewicz-Kurylonek N, Górska M, Kretowski A. Padilla-Martinez F, et al. Int J Mol Sci. 2022 Dec 16;23(24):16081. doi: 10.3390/ijms232416081. Int J Mol Sci. 2022. PMID: 36555722 Free PMC article. - Reducing Glycemic Indicators with Moderate Intensity Stepping of Varied, Short Durations in People with Pre-Diabetes.
Bartholomae E, Johnson Z, Moore J, Ward K, Kressler J. Bartholomae E, et al. J Sports Sci Med. 2018 Nov 20;17(4):680-685. eCollection 2018 Dec. J Sports Sci Med. 2018. PMID: 30479538 Free PMC article. Clinical Trial. - Mining the A.E. Watkins Wheat Landrace Collection for Variation in Starch Digestibility Using a New High-Throughput Assay.
Zafeiriou P, Savva GM, Ahn-Jarvis JH, Warren FJ, Pasquariello M, Griffiths S, Seung D, Hazard BA. Zafeiriou P, et al. Foods. 2023 Jan 6;12(2):266. doi: 10.3390/foods12020266. Foods. 2023. PMID: 36673358 Free PMC article.
References
- Pi-Sunyer FX. The obesity epidemic: pathophysiology and consequences of obesity. Obes Res. 2002;10(Suppl. 2):97S–104S. - PubMed
- Li Z, Bowerman S, Heber D. Health ramifications of the obesity epidemic. Surg Clin North Am. 2005;85:681–701. - PubMed
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486–494. - PubMed
- Califf RM, Boolell M, Haffner SM, et al. Prevention of diabetes and cardiovascular disease in patients with impaired glucose tolerance: rationale and design of the Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research (NAVIGATOR) Trial. Am Heart J. 2008;156:623–632. - PubMed
- Roumen C, Corpeleijn E, Feskens EJ, Mensink M, Saris WH, Blaak EE. Impact of 3-year lifestyle intervention on postprandial glucose metabolism: the SLIM study. Diabet Med. 2008;25:597–605. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical