Autophagy in idiopathic pulmonary fibrosis - PubMed (original) (raw)
Autophagy in idiopathic pulmonary fibrosis
Avignat S Patel et al. PLoS One. 2012.
Abstract
Background: Autophagy is a basic cellular homeostatic process important to cell fate decisions under conditions of stress. Dysregulation of autophagy impacts numerous human diseases including cancer and chronic obstructive lung disease. This study investigates the role of autophagy in idiopathic pulmonary fibrosis.
Methods: Human lung tissues from patients with IPF were analyzed for autophagy markers and modulating proteins using western blotting, confocal microscopy and transmission electron microscopy. To study the effects of TGF-β(1) on autophagy, human lung fibroblasts were monitored by fluorescence microscopy and western blotting. In vivo experiments were done using the bleomycin-induced fibrosis mouse model.
Results: Lung tissues from IPF patients demonstrate evidence of decreased autophagic activity as assessed by LC3, p62 protein expression and immunofluorescence, and numbers of autophagosomes. TGF-β(1) inhibits autophagy in fibroblasts in vitro at least in part via activation of mTORC1; expression of TIGAR is also increased in response to TGF-β(1). In the bleomycin model of pulmonary fibrosis, rapamycin treatment is antifibrotic, and rapamycin also decreases expression of á-smooth muscle actin and fibronectin by fibroblasts in vitro. Inhibition of key regulators of autophagy, LC3 and beclin-1, leads to the opposite effect on fibroblast expression of á-smooth muscle actin and fibronectin.
Conclusion: Autophagy is not induced in pulmonary fibrosis despite activation of pathways known to promote autophagy. Impairment of autophagy by TGF-β(1) may represent a mechanism for the promotion of fibrogenesis in IPF.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
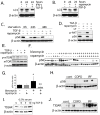
Figure 1. Autophagy is not increased in IPF.
A) IPF whole lung homogenate demonstrates increased ER stress (elevated XBP1 expression) and increased phosphorylation of AMPK, factors which should drive autophagy. B) LC3-II (lower band) expression in IPF whole lung homogenate is decreased relative to control lung tissue C) Densitometry of Western blots demonstrating LC3-II level is lower in IPF than in control lung (*p = 0.05). D) Increased p62 in IPF lung suggests decreased autophagy. E) Immunofluorescence confocal microscopy of control and IPF lung tissue for p62 (green), aggresome (red), DAPI (blue) demonstrates increased p62 expression and aggresomes. F) Representative electron microscopy images from IPF (panels A, B), control (Panel C), and COPD (panel D); white arrows indicate autophagosomes. G) Quantitation of autophagic vacuoles in control, IPF, and COPD lung by EM demonstrates significantly higher numbers in COPD (*p<0.05 for IPF vs. COPD).
Figure 2. TGF-β1 inhibits autophagy in vitro.
A) Human lung fibroblasts were cultured and treated with varying concentrations of TGF-β1. TGF-β1 inhibited activation of LC3, decreasing the intensity of the lower band on the western blot, and B) increased p62. C) Inhibition of LC3 activation by TGF-β1 with varying serum conditions. D) Fibroblasts treated with TGF-β1 for 24 hours and measurement of autophagic flux by LC3 western blotting (using lysosomal acidification inhibitor chloroquine). E) Densitometry of western blot shown in D (p = 0.045). F) Fluorescence microscopy of fibroblasts transfected with GFP-LC3 construct and stimulated with TGF-β1 showing that TGF-β1 inhibits formation of LC3 puncta. G) Confocal microscopy of type II alveolar epithelial cells isolated from GFP-LC3 transgenic mice and stimulated with TGF-β1 showing that TGF-β1 inhibits formation of LC3 puncta (green = GFP-LC3, red = SP-C, blue = DAPI). H) Quantification of GFP puncta per cell from figure 2G.
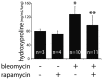
Figure 3. Effects of rapamycin on bleomycin induced fibrosis and autophagy.
Hydroxyproline assay measuring lung collagen content demonstrates co-administration of rapamycin and bleomycin protects against fibrosis (*p = 0.003 for control vs. bleomycin; **p = 0.008 for bleomycin vs. rapamycin + bleomycin).
Figure 4. TGF-β1 activates mTOR and TIGAR.
A) In human lung fibroblasts, TGF-β1 inhibits LC3-II formation, even in the presence of IFN-γ which induces autophagy. B) TGF-β1 is unable to inhibit LC3-II formation in presence of mTOR inhibitor rapamycin. C) TGF-β1 is able to activate mTORC1 which results in increased phospho-S6 but this activation does not occur in the presence of rapamycin. D) TGF-β1 appears to activate mTORC1 by activating upstream PI3K/AKT and treatment with PI3K inhibitor LY294002 prevents TGF-β1 induced mTOR activation. E) Western blot of phospho-mTOR (Ser2448) showing increased phospho-mTOR with TGF-β1 in fibroblasts and inhibition by rapamycin. F) Western blot of phospho-S6 from mouse lung tissue treated with bleomycin and rapamycin. G) Densitometry of blot from 4E. (*p = 0.03 for controls vs. bleomycin, **p = 0.003 for bleomycin vs rapamycin + bleomycin). H) phospho-S6 protein levels in human lung tissue is higher in IPF patients compared with COPD patients and healthy controls. I) TIGAR is induced by TGF-β1 in fibroblasts in a dose-responsive manner. J) Western blot demonstrating TIGAR protein levels in lung homogenate from human tissue is higher in IPF patients compared with COPD patients and healthy controls.
Figure 5. Inhibition of autophagy potentiates fibroblast activation.
A) Rapamycin inhibits fibronectin and α-SMA expression in fibroblasts, and abrogates TGF-β1 suppression of Id-1. B) Beclin and LC3 siRNA effectively inhibit protein expression. C) α-SMA and fibronectin expression increases in fibroblasts with beclin and LC3 silencing whereas collagen is unchanged. D) When LC3 is silenced, Id-1 expression is lower than control. E) Rapamycin does not modulate phosphorylation of Smad3 in response to TGF-β1.
Similar articles
- Tubastatin ameliorates pulmonary fibrosis by targeting the TGFβ-PI3K-Akt pathway.
Saito S, Zhuang Y, Shan B, Danchuk S, Luo F, Korfei M, Guenther A, Lasky JA. Saito S, et al. PLoS One. 2017 Oct 18;12(10):e0186615. doi: 10.1371/journal.pone.0186615. eCollection 2017. PLoS One. 2017. PMID: 29045477 Free PMC article. - Eupatilin inhibits pulmonary fibrosis by activating Sestrin2/PI3K/Akt/mTOR dependent autophagy pathway.
Gong H, Lyu X, Liu Y, Peng N, Tan S, Dong L, Zhang X. Gong H, et al. Life Sci. 2023 Dec 1;334:122218. doi: 10.1016/j.lfs.2023.122218. Epub 2023 Nov 1. Life Sci. 2023. PMID: 37918625 - Novel Mechanisms for the Antifibrotic Action of Nintedanib.
Rangarajan S, Kurundkar A, Kurundkar D, Bernard K, Sanders YY, Ding Q, Antony VB, Zhang J, Zmijewski J, Thannickal VJ. Rangarajan S, et al. Am J Respir Cell Mol Biol. 2016 Jan;54(1):51-9. doi: 10.1165/rcmb.2014-0445OC. Am J Respir Cell Mol Biol. 2016. PMID: 26072676 Free PMC article. - Updates on the controversial roles of regulatory lymphoid cells in idiopathic pulmonary fibrosis.
Curioni AV, Borie R, Crestani B, Helou DG. Curioni AV, et al. Front Immunol. 2024 Sep 25;15:1466901. doi: 10.3389/fimmu.2024.1466901. eCollection 2024. Front Immunol. 2024. PMID: 39386201 Free PMC article. Review. - Autophagy and inflammation in chronic respiratory disease.
Racanelli AC, Kikkers SA, Choi AMK, Cloonan SM. Racanelli AC, et al. Autophagy. 2018;14(2):221-232. doi: 10.1080/15548627.2017.1389823. Epub 2018 Feb 8. Autophagy. 2018. PMID: 29130366 Free PMC article. Review.
Cited by
- Essential role for the ATG4B protease and autophagy in bleomycin-induced pulmonary fibrosis.
Cabrera S, Maciel M, Herrera I, Nava T, Vergara F, Gaxiola M, López-Otín C, Selman M, Pardo A. Cabrera S, et al. Autophagy. 2015 Apr 3;11(4):670-84. doi: 10.1080/15548627.2015.1034409. Autophagy. 2015. PMID: 25906080 Free PMC article. - Berberine inhibits Smad and non-Smad signaling cascades and enhances autophagy against pulmonary fibrosis.
Chitra P, Saiprasad G, Manikandan R, Sudhandiran G. Chitra P, et al. J Mol Med (Berl). 2015 Sep;93(9):1015-31. doi: 10.1007/s00109-015-1283-1. Epub 2015 Apr 17. J Mol Med (Berl). 2015. PMID: 25877860 - Cellular and Molecular Mechanisms in Idiopathic Pulmonary Fibrosis.
Zhang Y, Wang J. Zhang Y, et al. Adv Respir Med. 2023 Jan 31;91(1):26-48. doi: 10.3390/arm91010005. Adv Respir Med. 2023. PMID: 36825939 Free PMC article. Review. - Immunomodulatory role of azithromycin: Potential applications to radiation-induced lung injury.
Yan Y, Wu L, Li X, Zhao L, Xu Y. Yan Y, et al. Front Oncol. 2023 Mar 8;13:966060. doi: 10.3389/fonc.2023.966060. eCollection 2023. Front Oncol. 2023. PMID: 36969016 Free PMC article. Review. - Mesenchymal Stem Cells Attenuate Diabetic Lung Fibrosis via Adjusting Sirt3-Mediated Stress Responses in Rats.
Chen Y, Zhang F, Wang D, Li L, Si H, Wang C, Liu J, Chen Y, Cheng J, Lu Y. Chen Y, et al. Oxid Med Cell Longev. 2020 Feb 4;2020:8076105. doi: 10.1155/2020/8076105. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32089781 Free PMC article.
References
- Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. - PubMed
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. - PubMed
- Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL087122/HL/NHLBI NIH HHS/United States
- T32 HL007633/HL/NHLBI NIH HHS/United States
- 5T32HL007633-25/HL/NHLBI NIH HHS/United States
- K23 HL087030/HL/NHLBI NIH HHS/United States
- 5T32HL007633-24/HL/NHLBI NIH HHS/United States
- R01HL087122-01/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials