"Redundancy" of endocannabinoid inactivation: new challenges and opportunities for pain control - PubMed (original) (raw)
Review
. 2012 May 16;3(5):356-63.
doi: 10.1021/cn300015x. Epub 2012 Feb 27.
Affiliations
- PMID: 22860203
- PMCID: PMC3382450
- DOI: 10.1021/cn300015x
Review
"Redundancy" of endocannabinoid inactivation: new challenges and opportunities for pain control
Fabiana Piscitelli et al. ACS Chem Neurosci. 2012.
Abstract
Redundancy of metabolic pathways and molecular targets is a typical feature of all lipid mediators, and endocannabinoids, which were originally defined as endogenous agonists at cannabinoid CB(1) and CB(2) receptors, are no exception. In particular, the two most studied endocannabinoids, anandamide and 2-arachidonoylglycerol, are inactivated through alternative biochemical routes, including hydrolysis and oxidation, and more than one enzyme might be used even for the same type of inactivating reaction. These enzymes also recognize as substrates other concurrent lipid mediators, whereas, in turn, endocannabinoids might interact with noncannabinoid receptors with subcellular distribution and ultimate biological actions either similar to or completely different from those of cannabinoid receptors. Even splicing variants of endocannabinoid hydrolyzing enzymes, such as FAAH-1, might play distinct roles in endocannabinoid inactivation. Finally, the products of endocannabinoid catabolism may have their own targets, with biological roles different from those of cannabinoid receptors. These peculiarities of endocannabinoid signaling have complicated the use of inhibitors of its inactivation mechanisms as a safer and more efficacious alternative to the direct targeting of cannabinoid receptors for the treatment of several pathological conditions, including pain. However, new strategies, including the rediscovery of "dirty drugs", and the use of certain natural products (including non-THC cannabis constituents), are emerging that might allow us to make a virtue of necessity and exploit endocannabinoid redundancy to develop new analgesics.
Keywords: COX-2; FAAH-1; MAGL; TRPA1; TRPV1; cannabinoid; receptor.
Figures
Figure 1
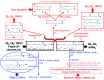
Redundancy of endocannabinoid receptors, catabolic pathways, and enzymes. Hydrolytic (in blue) and oxidative (in red) metabolism of the two most studied endocannabinoids, anandamide (AEA) and 2-arachidonoylglycerol (2-AG). Fatty acid amide hydrolase-1 (FAAH-1) and -2 (FAAH-2), as well as _N_-acylethanolamine hydrolyzing acid amidase (NAAA) catalyze, with varying specificity, the hydrolysis to ethanolamine and the corresponding fatty acids of various _N_-acylethanolamines, including _N_-oleoyl-ethanolamine (OEA) and _N_-palmitoyl-ethanolamine (PEA), which are only weakly active per se on cannabinoid receptors but can stimulate transient receptor potential vanilloid type-1 (TRPV1) and/or peroxisome proliferator-activated receptor-α (PPAR-α). Also AEA and 2-AG have been shown to interact with noncannabinoid receptors. Monoacylglycerol lipase (MAGL), and to a minor extent, α,β-hydrolase-6 (ABHD6) and α,β-hydrolase-12 (ABHD12), as well as FAAH-1, catalyze the hydrolysis of 2-AG. MAGL-catalyzed formation of arachidonic acid (AA) can be utilized as an alternative to phospholipase A2-mediated pathways to lead, using enzymes of the AA cascade, to the formation of eicosanoids, which then act at their own specific receptors. These same enzymes can be used to oxidize endocannabinoids to the corresponding hydroxy (in the case of lipoxygenases) and epoxy (in the case of cytochrome p450 monooxidases)-derivatives, or to prostamides and prostaglandin glycerol esters (in the case of cycloxygenase-2 and prostaglandin synthases). The formation of hydroxyl and epoxy derivatives, which still exhibit activity at cannabinoid receptors and TRPV1 (or other TRP) channels, has been so far shown to occur only in vitro. Prostamide F2α and prostaglandin E2-glycerol ester, instead, were recently reported to occur in the spinal cord of mice with knee inflammation and paw skin of rats with local inflammation, respectively, and are inactive at cannabinoid, FP or EP receptors. They were suggested to act at new receptors. Legend: R, ethanolamine or glycerol group.
Figure 2
Paracetamol and metamizol are metabolized in vivo to compounds with multitarget analgesic action. Fatty acid amide hydrolase-1 (FAAH-1) catalyzes the condensation of arachidonic acid (AA) with amines produced in vivo from the metabolism of acetaminophen (a) or dipyrone (b) (i.e., _p_-aminophenol and compounds 2 and 3, respectively). The resulting metabolites, i.e., AM404 and the arachidonoylamides of 4-methylaminoantipyrine (5) and 4-aminoantipyrine (6), may have different targets involved in pain control. They may still inhibit cycloxygenase-1 (COX-1) and -2 (COX-2) (as in the case of AM404 and compound 5), and interact with transient receptor potential vanilloid type-1 (TRPV1) channels (by activating and desensitizing them, as in the case of AM404, or by antagonizing them, as in the case of compound 5). Compounds 5 and 6 also weakly bind to cannabinoid CB1 and CB2 receptors. AM404 was also described to weakly inhibit FAAH-1 and bind to CB1 receptors (not shown), and to counteract endocannabinoid cellular uptake. Finally, other in vivo metabolites of acetaminophen potently activate transient receptor potential ankyrin type-1 (TRPA1) channels and were suggested to cause analgesia through the subsequent inactivation of voltage-activated calcioum channels in spinal neurons.
Figure 3
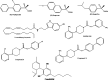
Chemical structures of some synthetic or natural multitarget analgesics discussed in this review.
Figure 4
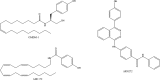
Chemical structures of some synthetic inhibitors of anandamide cellular uptake discussed in this review.
Similar articles
- The Endocannabinoid System Modulating Levels of Consciousness, Emotions and Likely Dream Contents.
Murillo-Rodriguez E, Pastrana-Trejo JC, Salas-Crisóstomo M, de-la-Cruz M. Murillo-Rodriguez E, et al. CNS Neurol Disord Drug Targets. 2017;16(4):370-379. doi: 10.2174/1871527316666170223161908. CNS Neurol Disord Drug Targets. 2017. PMID: 28240187 Review. - Non-psychotropic analgesic drugs from the endocannabinoid system: "magic bullet" or "multiple-target" strategies?
Starowicz K, Di Marzo V. Starowicz K, et al. Eur J Pharmacol. 2013 Sep 15;716(1-3):41-53. doi: 10.1016/j.ejphar.2013.01.075. Epub 2013 Mar 13. Eur J Pharmacol. 2013. PMID: 23500197 Review. - Druggable Targets in Endocannabinoid Signaling.
Gregus AM, Buczynski MW. Gregus AM, et al. Adv Exp Med Biol. 2020;1274:177-201. doi: 10.1007/978-3-030-50621-6_8. Adv Exp Med Biol. 2020. PMID: 32894511 Free PMC article. Review. - FAAH and MAGL inhibitors: therapeutic opportunities from regulating endocannabinoid levels.
Petrosino S, Di Marzo V. Petrosino S, et al. Curr Opin Investig Drugs. 2010 Jan;11(1):51-62. Curr Opin Investig Drugs. 2010. PMID: 20047159 Review.
Cited by
- Dual-Acting Compounds Targeting Endocannabinoid and Endovanilloid Systems-A Novel Treatment Option for Chronic Pain Management.
Malek N, Starowicz K. Malek N, et al. Front Pharmacol. 2016 Aug 17;7:257. doi: 10.3389/fphar.2016.00257. eCollection 2016. Front Pharmacol. 2016. PMID: 27582708 Free PMC article. Review. - Transient receptor potential ion channels in primary sensory neurons as targets for novel analgesics.
Sousa-Valente J, Andreou AP, Urban L, Nagy I. Sousa-Valente J, et al. Br J Pharmacol. 2014 May;171(10):2508-27. doi: 10.1111/bph.12532. Br J Pharmacol. 2014. PMID: 24283624 Free PMC article. Review. - The role of dopamine and endocannabinoid systems in prefrontal cortex development: Adolescence as a critical period.
Peters KZ, Naneix F. Peters KZ, et al. Front Neural Circuits. 2022 Nov 1;16:939235. doi: 10.3389/fncir.2022.939235. eCollection 2022. Front Neural Circuits. 2022. PMID: 36389180 Free PMC article. Review. - Implication of the anti-inflammatory bioactive lipid prostaglandin D2-glycerol ester in the control of macrophage activation and inflammation by ABHD6.
Alhouayek M, Masquelier J, Cani PD, Lambert DM, Muccioli GG. Alhouayek M, et al. Proc Natl Acad Sci U S A. 2013 Oct 22;110(43):17558-63. doi: 10.1073/pnas.1314017110. Epub 2013 Oct 7. Proc Natl Acad Sci U S A. 2013. PMID: 24101490 Free PMC article. - Celastrol attenuates inflammatory and neuropathic pain mediated by cannabinoid receptor type 2.
Yang L, Li Y, Ren J, Zhu C, Fu J, Lin D, Qiu Y. Yang L, et al. Int J Mol Sci. 2014 Aug 6;15(8):13637-48. doi: 10.3390/ijms150813637. Int J Mol Sci. 2014. PMID: 25101848 Free PMC article.
References
- Hosking R. D.; Zajicek J. P. (2008) Therapeutic potential of cannabis in pain medicine. Br. J. Anaesth. 101, 59–68. - PubMed
- Matsuda L. A.; Lolait S. J.; Brownstein M. J.; Young A. C.; Bonner T. I. (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564. - PubMed
- Munro S.; Thomas K. L.; Abu-Shaar M. (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365, 61–65. - PubMed
- Di Marzo V.; Fontana A. (1995) Anandamide, an endogenous cannabinomimetic eicosanoid: ’killing two birds with one stone’. Prostaglandins, Leukotrienes Essent. Fatty Acids 53, 1–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials