A review of the human clinical studies involving Citrus aurantium (bitter orange) extract and its primary protoalkaloid p-synephrine - PubMed (original) (raw)
Review
A review of the human clinical studies involving Citrus aurantium (bitter orange) extract and its primary protoalkaloid p-synephrine
Sidney J Stohs et al. Int J Med Sci. 2012.
Abstract
This review summarizes the published as well as unpublished human studies involving Citrus aurantium (bitter orange) extract and its primary protoalkaloid p-synephrine, providing information and an assessment of the safety and efficacy of these widely used products. The results of over 20 studies involving a total of approximately 360 subjects that consumed p-synephrine alone or in combination with other ingredients are reviewed and critiqued. Over 50 % of the subjects involved in these studies were overweight/obese, and approximately two-thirds of these overweight/obese subjects consumed caffeine (132-528 mg/day) in conjunction with p-synephrine (10-53 mg/day). Bitter orange/p-synephrine containing products were consumed for up to 12 weeks. Approximately 44 % of the subjects consumed a bitter orange/p-synephrine only product, while the remainder consumed a complex product that contained multiple ingredients in addition to p-synephrine. In general, bitter orange extract alone (p-synephrine) or in combination with other herbal ingredients did not produce significant adverse events as an increase in heart rate or blood pressure, or alter electrocardiographic data, serum chemistry, blood cell counts or urinalysis. p-Synephrine alone as well as in combination products were shown to increase resting metabolic rate and energy expenditure, and modest increases in weight loss were observed with bitter orange extract/p-synephrine-containing products when given for six to 12 weeks. Longer term studies are needed to further assess the efficacy of these products and affirm their safety under these conditions.
Keywords: Citrus aurantium; bitter orange; caffeine.; efficacy; human subjects; obesity/overweight; p-synephrine; safety.
Conflict of interest statement
Competing Interests: See Acknowledgements. Authors declare they have no other competing interest.
Figures
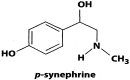
Fig 1
Chemical structure of _p_-synephrine.
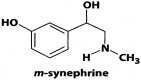
Fig 2
Chemical structure of _m_-synephrine.
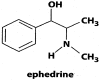
Fig 3
Chemical structure of ephedrine
Similar articles
- The safety of Citrus aurantium (bitter orange) and its primary protoalkaloid p-synephrine.
Stohs SJ, Preuss HG, Shara M. Stohs SJ, et al. Phytother Res. 2011 Oct;25(10):1421-8. doi: 10.1002/ptr.3490. Epub 2011 Apr 8. Phytother Res. 2011. PMID: 21480414 Review. - A 60day double-blind, placebo-controlled safety study involving Citrus aurantium (bitter orange) extract.
Kaats GR, Miller H, Preuss HG, Stohs SJ. Kaats GR, et al. Food Chem Toxicol. 2013 May;55:358-62. doi: 10.1016/j.fct.2013.01.013. Epub 2013 Jan 25. Food Chem Toxicol. 2013. PMID: 23354394 - Safety, Efficacy, and Mechanistic Studies Regarding Citrus aurantium (Bitter Orange) Extract and p-Synephrine.
Stohs SJ. Stohs SJ. Phytother Res. 2017 Oct;31(10):1463-1474. doi: 10.1002/ptr.5879. Epub 2017 Jul 28. Phytother Res. 2017. PMID: 28752649 Free PMC article. Review. - Cardiovascular Safety of Oral p-Synephrine (Bitter Orange) in Healthy Subjects: A Randomized Placebo-Controlled Cross-over Clinical Trial.
Shara M, Stohs SJ, Mukattash TL. Shara M, et al. Phytother Res. 2016 May;30(5):842-7. doi: 10.1002/ptr.5590. Epub 2016 Mar 7. Phytother Res. 2016. PMID: 26948284 Clinical Trial. - Safety evaluation of p-synephrine following 15 days of oral administration to healthy subjects: A clinical study.
Shara M, Stohs SJ, Smadi MM. Shara M, et al. Phytother Res. 2018 Jan;32(1):125-131. doi: 10.1002/ptr.5956. Epub 2017 Nov 12. Phytother Res. 2018. PMID: 29130542
Cited by
- Non-invasive assessment of proarrhythmic risks associated with isoprenaline and the dietary supplement ingredient synephrine using human induced pluripotent stem cell-derived cardiomyocytes.
Yuan X, Yu T, Zhang Z, Li S. Yuan X, et al. Front Cardiovasc Med. 2024 Jun 7;11:1407138. doi: 10.3389/fcvm.2024.1407138. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38911513 Free PMC article. - Citrus aurantium L. and synephrine improve brown adipose tissue function in adolescent mice programmed by early postnatal overfeeding.
Guimarães AC, de Moura EG, Silva SG, Lopes BP, Bertasso IM, Pietrobon CB, Quitete FT, de Oliveira Malafaia T, Souza ÉPG, Lisboa PC, de Oliveira E. Guimarães AC, et al. Front Nutr. 2024 Jan 11;10:1278121. doi: 10.3389/fnut.2023.1278121. eCollection 2023. Front Nutr. 2024. PMID: 38274208 Free PMC article. - Synephrine and Its Derivative Compound A: Common and Specific Biological Effects.
Dodonova SA, Zhidkova EM, Kryukov AA, Valiev TT, Kirsanov KI, Kulikov EP, Budunova IV, Yakubovskaya MG, Lesovaya EA. Dodonova SA, et al. Int J Mol Sci. 2023 Dec 15;24(24):17537. doi: 10.3390/ijms242417537. Int J Mol Sci. 2023. PMID: 38139366 Free PMC article. Review. - Notifications and Health Consequences of Unauthorized Pharmaceuticals in Food Supplements.
Amidžić M, Banović Fuentes J, Banović J, Torović L. Amidžić M, et al. Pharmacy (Basel). 2023 Sep 23;11(5):154. doi: 10.3390/pharmacy11050154. Pharmacy (Basel). 2023. PMID: 37888499 Free PMC article. - Roles of citrus fruits on energy expenditure, body weight management, and metabolic biomarkers: a comprehensive review.
Aslan MN, Sukan-Karaçağıl B, Acar-Tek N. Aslan MN, et al. Nutr Rev. 2024 Sep 1;82(9):1292-1307. doi: 10.1093/nutrit/nuad116. Nutr Rev. 2024. PMID: 37702528 Free PMC article. Review.
References
- Chen JK, Chen TT. Zhi Shi (Fructus Aurantii Immaturus). Chinese Medical Herbology and Pharmacology. City of Industry, CA USA: Art of Medicine Press; 2004. pp. 485–487.
- Stohs SJ, Shara M. A review of the safety and efficacy of Citrus aurantium in weight management. In: Bagchi D, Preuss HG, editors. Obesity: Epidemiology, Pathophysiology, and Prevention. Boca Raton, FL, USA: CRC Press; 2007. pp. 371–382.
- Pellati F, Benvenuti S. Chromatographic and electrophoretic methods for the analysis of phenethylamine alkaloids in Citrus aurantium. J Chromatog A. 2007;1171:71–88. - PubMed
- Sander LC, Putzbach K, Nelson BC. et al. Certification of standard reference materials containing bitter orange. Analyt Bioanalyt Chem. 2008;391:2023–2034. - PubMed
- Evans RL, Pho AN, Roman MC, Betz JM. Certification of standard reference materials containing bitter orange. Analyt Bioanalyt Chem. 2008;391:2023–2034. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical