Isorhamnetin inhibits proliferation and invasion and induces apoptosis through the modulation of peroxisome proliferator-activated receptor γ activation pathway in gastric cancer - PubMed (original) (raw)
. 2012 Nov 2;287(45):38028-40.
doi: 10.1074/jbc.M112.388702. Epub 2012 Sep 19.
Kanjoormana Aryan Manu, Muthu K Shanmugam, Feng Li, Kodappully Sivaraman Siveen, Shireen Vali, Shweta Kapoor, Taher Abbasi, Rohit Surana, Duane T Smoot, Hassan Ashktorab, Patrick Tan, Kwang Seok Ahn, Chun Wei Yap, Alan Prem Kumar, Gautam Sethi
Affiliations
- PMID: 22992727
- PMCID: PMC3488073
- DOI: 10.1074/jbc.M112.388702
Isorhamnetin inhibits proliferation and invasion and induces apoptosis through the modulation of peroxisome proliferator-activated receptor γ activation pathway in gastric cancer
Lalitha Ramachandran et al. J Biol Chem. 2012.
Erratum in
- J Biol Chem. 2013 Jun 28;288(26):18777
Abstract
Gastric cancer (GC) is a lethal malignancy and the second most common cause of cancer-related deaths. Although treatment options such as chemotherapy, radiotherapy, and surgery have led to a decline in the mortality rate due to GC, chemoresistance remains as one of the major causes for poor prognosis and high recurrence rate. In this study, we investigated the potential effects of isorhamnetin (IH), a 3'-O-methylated metabolite of quercetin on the peroxisome proliferator-activated receptor γ (PPAR-γ) signaling cascade using proteomics technology platform, GC cell lines, and xenograft mice model. We observed that IH exerted a strong antiproliferative effect and increased cytotoxicity in combination with chemotherapeutic drugs. IH also inhibited the migratory/invasive properties of GC cells, which could be reversed in the presence of PPAR-γ inhibitor. We found that IH increased PPAR-γ activity and modulated the expression of PPAR-γ regulated genes in GC cells. Also, the increase in PPAR-γ activity was reversed in the presence of PPAR-γ-specific inhibitor and a mutated PPAR-γ dominant negative plasmid, supporting our hypothesis that IH can act as a ligand of PPAR-γ. Using molecular docking analysis, we demonstrate that IH formed interactions with seven polar residues and six nonpolar residues within the ligand-binding pocket of PPAR-γ that are reported to be critical for its activity and could competitively bind to PPAR-γ. IH significantly increased the expression of PPAR-γ in tumor tissues obtained from xenograft model of GC. Overall, our findings clearly indicate that antitumor effects of IH may be mediated through modulation of the PPAR-γ activation pathway in GC.
Figures
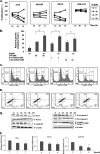
FIGURE 1.
A, the chemical structure of IH. B, predictive in silico virtual tumor cell platform generated results. The figure illustrates a high level view of the maze of interactions and cross-talks present in the virtual tumor cell platform. The Cellworks virtual epithelial tumor cell platform on which predictive studies were conducted is an integrated representation of the pathways in cancer that includes phenotypes of proliferation, apoptosis, angiogenesis, metastasis, and conditions of tumor microenvironment such as tumor-associated inflammation. The set of graphs here demonstrate the effect on biomarkers upon treatment with IH. IH was shown as a PPAR-γ agonist in the system and tested on the AGS baseline at 0.5 and 5 μ
m
with a Ka of 1.19 μ
m
. C, effect on increase in PPAR-γ activity with IH. PPARG shows increases of 1.36- and 2.22-fold, respectively, with 0.5 and 5 μ
m
of IH. D, the figure shows the effect of IH on survival markers-Bcl-2, Bcl-xL, survivin, and Mcl-1. Survivin and myeloid cell leukemia sequence 1 (Bcl-2-related) show a higher reduction with IH varying from 50–60% as compared with Bcl-2 and Bcl-xL where we see a reduction of ∼20–30%. E, the figure depicts the impact of IH on proliferative markers-CCND1 (Cyclin D1) and CCNE (Cyclin E). CCND1 is showing almost a 90% reduction with 5 μ
m
of IH, and CCNE is showing a reduction of 40 and 60%, respectively, at the two dosages for IH. F, the figure shows the impact of IH on angiogenic and metastatic markers VEGFA and CXCR4. CXCR4 is showing a reduction of ∼38 and ∼58% with 0.5 and 5 μ
m
of IH, and VEGFA shows a reduction of 35 and 45%, respectively. G, the impact of IH on apoptotic markers: active CASP3 and CASP9. CASP9 is showing a higher increase of 500% with IH treatment as compared with CASP3, which is showing a 200% increase with IH. H, the impact of IH on BAX and BAK levels. BAK is showing an increase of 175 and 340% with 0.5 and 5 μ
m
of the drug. The increase in BAX is only ∼50%. I, the effect of IH on cleaved PARP1. PARP1 cleaved is showing an increase of 2500 and 5000% with 0.5 and 5 μ
m
of IH, respectively.
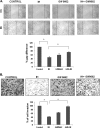
FIGURE 2.
A, antiproliferative effects of IH in AGS, MKN45, SNU5 gastric cancer cells, and HFE-145 gastric epithelial cells. Cell viability was determined by MTT assay and is reported as the percentage of viable cells relative to the control. The values are the means ± S.E. of three independent experiments. B, IH potentiates the effect of various chemotherapeutic drugs (5-fluorouracil, doxorubicin, and capacetabine) in the AGS cell line significantly. AGS cells were treated with either 10 μ
m
IH alone or in combination with various chemotherapeutic drugs for 24 h before MTT solution was added. The data were expressed as the percentages of dead cells relative to the control. The values are the means ± S.E. of three independent experiments. *, p < 0.05. C, time-dependent effects of IH on cell cycle distribution in AGS cells. The cells were exposed to 25 μ
m
of IH for 12, 24, and 48 h followed by propidium iodide staining. The data are representative of three independent experiments. D, IH induces apoptosis in a time-dependent manner as observed by annexin V staining. The data are representative of three independent experiments. E, Western blot analysis of various gene products upon IH treatment. AGS cells were treated with 25 μ
m
IH for 6, 12, 24, and 48 h. Whole cell extracts were resolved on SDS-PAGE and probed with the indicated antibodies. The data are representative of at least three independent experiments. F, AGS cells were treated with 25 μ
m
IH for the indicated time intervals, after which cells were harvested, and RNA samples were extracted. 1-μg portions of the respective RNA extracts were subjected to reverse transcription to generate corresponding cDNA. Real time PCR was performed to measure the relative quantities of mRNA. Each RT product was targeted against Bcl-2, Bcl-xL, and cyclin D1 TaqMan probes, with HuGAPDH as endogenous control for measurement of equal loading of RNA samples. The results were analyzed using Sequence Detection Software version 1.3 provided by Applied Biosystems. *, p < 0.05; **, p < 0.01.
FIGURE 3.
A, microscopic observation of the migration of AGS cells after pretreatment with GW9662 (20 μ
m
for 2 h), followed by incubation with IH for 8 h. The cells were also treated alone with 25 μ
m
IH for 8 h and GW9662 (20 μ
m
for 2 h). The data are representative of three independent experiments. B, the cell invasion assay for evaluating the inhibitory effect of IH on gastric cancer cell invasion after pretreatment with GW9662 (20 μ
m
for 2 h), followed by incubation with IH for 8 h. The cells were also treated alone with 25 μ
m
IH for 8 h and GW9662 (20 μ
m
for 2 h). The cells were fixed with 4% paraformaldehyde before staining with 0.5% crystal violet as described under “Experimental Procedures.” The percentage of the migratory cells of the treated group was normalized against the untreated group. The values are the means ± S.E. of two or three independent experiments. *, p < 0.05.
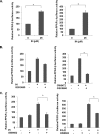
FIGURE 4.
Effect of IH PPAR activity in GC cells. A, effect of IH in PPARs. The cells were transfected with GAL4-PPAR-β/δ LBD and GAL4-PPAR-γ LBD plasmids, together with GAL4-Luc and β-gal plasmids for 4 h before treatment with 25 μ
m
IH for 18 h. The data are expressed as percentages of the respective PPAR activity relative to the control. The values are the means ± S.E. of two or three independent experiments. *, p < 0.05. B, the inhibitor of PPAR-β/δ, GSK0660, could not block IH-induced PPAR-β/δ activity. The cells were transfected with GAL4-PPAR-β/δ LBD plasmids together with GAL4-Luc and β-gal plasmid for 4 h. The cells were pretreated with 50 μ
m
GSK0660 for 4 h before treatment with 25 μ
m
IH or 10 μ
m
GW0742, a PPAR-β/δ agonist, both for 18 h. The data are expressed as percentages of the PPAR-β/δ activity relative to the control. The values are the means ± S.E. of two or three independent experiments. *, p < 0.05. C, IH-induced PPAR-γ activity could be blocked by GW9662, an inhibitor of PPAR-γ. The cells were transfected with GAL4-PPAR-γ LBD plasmids together with GAL4-Luc and β-gal plasmid for 4 h. The cells were pretreated with 10 μ
m
or 20 μ
m
GW9662 for 2 h before treatment with 25 μ
m
IH or 20 μ
m
PGJ2, a PPAR-γ agonist, both for 18 h. The data are expressed as percentages of the PPAR-γ activity relative to the control. The values are the means ± S.E. of two or three independent experiments. *, p < 0.05.
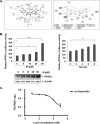
FIGURE 5.
A, the ligand interaction map of IH inside PPAR-γ (left panel) and three-dimensional conformational structure of IH inside PPAR-γ (right panel). B, IH increases PPAR-γ activity in a dose dependent manner, also shown by Western blot (left panel). IH also increases PPAR-γ activity in a time-dependent manner (right panel). The cells were transfected with pPPRE-tk-Luc and β-gal plasmid for 4 h before treatment with the indicated concentrations of IH. The data are expressed as percentages of the PPAR-γ activity relative to the control. The values are the means ± S.E. of two or three independent experiments. *, p < 0.05; **, p < 0.01. C, in vitro competitive binding assay showed that IH could bind competitively to PPAR-γ. Serial dilutions of IH (1% final Me2SO concentration, serial dilutions performed in 100% Me2SO) were prepared in a 384-well polypropylene assay plate. FluormoneTM Pan-PPAR Green, PPAR-γ-LBD, and Tb-anti-GST Ab were then added to each sample well as described in the protocol. The assay mixture was incubated for 1 h at room temperature prior to measuring the 520-nm/490-nm emission ratio of each well. The error bars represent the S.E. of duplicate wells (n = 2).
FIGURE 6.
IH-induced PPAR-γ activity could be blocked by GW9662, an inhibitor of PPAR-γ. A, GW9662 partially reverses IH-induced apoptosis in AGS cells tested by annexin V staining. Western blot denotes the efficiency of transfection. The cells were pretreated with 10 or 20 μ
m
of GW9662 for 2 h before treatment with 25 μ
m
of IH for 12 h. The cells were then harvested and stained with annexin V-PI as described under “Experimental Procedures.” The values are the means ± S.E. of three independent experiments. *, p < 0.05. B, dominant negative PPAR-γ reverses IH-induced PPAR-γ activity. Western blot denotes the efficiency of transfection. The cells were transfected with PPAR-γDN or pCMX plasmids, together with pPPRE-tk-Luc and β-gal plasmids for 4 h before treatment with 25 μ
m
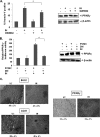
of IH for 18 h. The data are expressed as percentages of the PPAR-γ activity relative to the control. The values are means ± S.E. of two or three independent experiments. *, p < 0.05. C, immunohistochemical analysis of Bcl-2, CD31, and PPAR-γ showed the increase in the expression of PPAR-γ and the inhibition in Bcl-2 and CD31 expression in IH-treated samples as compared with control group. The percentage indicates positive staining for the given biomarker. The photographs were taken at the magnification of 20×.
Similar articles
- Isorhamnetin augments the anti-tumor effect of capecitabine through the negative regulation of NF-κB signaling cascade in gastric cancer.
Manu KA, Shanmugam MK, Ramachandran L, Li F, Siveen KS, Chinnathambi A, Zayed ME, Alharbi SA, Arfuso F, Kumar AP, Ahn KS, Sethi G. Manu KA, et al. Cancer Lett. 2015 Jul 10;363(1):28-36. doi: 10.1016/j.canlet.2015.03.033. Epub 2015 Mar 28. Cancer Lett. 2015. PMID: 25827070 - Honokiol inhibits gastric tumourigenesis by activation of 15-lipoxygenase-1 and consequent inhibition of peroxisome proliferator-activated receptor-gamma and COX-2-dependent signals.
Liu SH, Shen CC, Yi YC, Tsai JJ, Wang CC, Chueh JT, Lin KL, Lee TC, Pan HC, Sheu ML. Liu SH, et al. Br J Pharmacol. 2010 Aug;160(8):1963-72. doi: 10.1111/j.1476-5381.2010.00804.x. Br J Pharmacol. 2010. PMID: 20649594 Free PMC article. - Anticancer activity of thymoquinone in breast cancer cells: possible involvement of PPAR-γ pathway.
Woo CC, Loo SY, Gee V, Yap CW, Sethi G, Kumar AP, Tan KH. Woo CC, et al. Biochem Pharmacol. 2011 Sep 1;82(5):464-75. doi: 10.1016/j.bcp.2011.05.030. Epub 2011 Jun 14. Biochem Pharmacol. 2011. PMID: 21679698 - Molecular cross-regulation between PPAR-γ and other signaling pathways: implications for lung cancer therapy.
Reka AK, Goswami MT, Krishnapuram R, Standiford TJ, Keshamouni VG. Reka AK, et al. Lung Cancer. 2011 May;72(2):154-9. doi: 10.1016/j.lungcan.2011.01.019. Epub 2011 Feb 26. Lung Cancer. 2011. PMID: 21354647 Free PMC article. Review. - Flavonoids and Gastric Cancer Therapy: From Signaling Pathway to Therapeutic Significance.
Cai J, Tan X, Hu Q, Pan H, Zhao M, Guo C, Zeng J, Ma X, Zhao Y. Cai J, et al. Drug Des Devel Ther. 2024 Jul 25;18:3233-3253. doi: 10.2147/DDDT.S466470. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39081701 Free PMC article. Review.
Cited by
- Natural Polyphenols for Prevention and Treatment of Cancer.
Zhou Y, Zheng J, Li Y, Xu DP, Li S, Chen YM, Li HB. Zhou Y, et al. Nutrients. 2016 Aug 22;8(8):515. doi: 10.3390/nu8080515. Nutrients. 2016. PMID: 27556486 Free PMC article. Review. - PPARγ induces growth inhibition and apoptosis through upregulation of insulin-like growth factor-binding protein-3 in gastric cancer cells.
Kim SY, Kim MS, Lee MK, Kim JS, Yi HK, Nam SY, Lee DY, Hwang PH. Kim SY, et al. Braz J Med Biol Res. 2015 Mar;48(3):226-33. doi: 10.1590/1414-431X20144212. Epub 2015 Jan 13. Braz J Med Biol Res. 2015. PMID: 25590353 Free PMC article. - Escaping mechanisms of ESKAPE pathogens from antibiotics and their targeting by natural compounds.
Jadimurthy R, Mayegowda SB, Nayak SC, Mohan CD, Rangappa KS. Jadimurthy R, et al. Biotechnol Rep (Amst). 2022 Apr 4;34:e00728. doi: 10.1016/j.btre.2022.e00728. eCollection 2022 Jun. Biotechnol Rep (Amst). 2022. PMID: 35686013 Free PMC article. - Association of the Epithelial-Mesenchymal Transition (EMT) with Cisplatin Resistance.
Ashrafizadeh M, Zarrabi A, Hushmandi K, Kalantari M, Mohammadinejad R, Javaheri T, Sethi G. Ashrafizadeh M, et al. Int J Mol Sci. 2020 Jun 3;21(11):4002. doi: 10.3390/ijms21114002. Int J Mol Sci. 2020. PMID: 32503307 Free PMC article. Review. - Impact of Phytochemicals on PPAR Receptors: Implications for Disease Treatments.
Enayati A, Ghojoghnejad M, Roufogalis BD, Maollem SA, Sahebkar A. Enayati A, et al. PPAR Res. 2022 Aug 31;2022:4714914. doi: 10.1155/2022/4714914. eCollection 2022. PPAR Res. 2022. PMID: 36092543 Free PMC article. Review.
References
- Ford A. C. (2011) Chemoprevention for gastric cancer. Best Pract. Res. Clin. Gastroenterol. 25, 581–592 - PubMed
- Yeoh K. G. (2007) How do we improve outcomes for gastric cancer? J. Gastroenterol. Hepatol. 22, 970–972 - PubMed
- Lochhead P., El-Omar E. M. (2008) Gastric cancer. Br. Med. Bull. 85, 87–100 - PubMed
- Roukos D. H., Kappas A. M. (2005) Perspectives in the treatment of gastric cancer. Nat. Clin. Pract. Oncol. 2, 98–107 - PubMed
- Ohtsu A. (2008) Chemotherapy for metastatic gastric cancer. Past, present, and future. Journal of gastroenterology 43, 256–264 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous