Evaluation of bone regeneration using the rat critical size calvarial defect - PubMed (original) (raw)
Evaluation of bone regeneration using the rat critical size calvarial defect
Patrick P Spicer et al. Nat Protoc. 2012 Oct.
Abstract
Animal models that are reliably reproducible, appropriate analogs to the clinical condition they are used to investigate, and that offer minimal morbidity and periprocedural mortality to the subject, are the keystone to the preclinical development of translational technologies. For bone tissue engineering, a number of small animal models exist. Here we describe the protocol for one such model, the rat calvarial defect. This versatile model allows for evaluation of biomaterials and bone tissue engineering approaches within a reproducible, non-load-bearing orthotopic site. Crucial steps for ensuring appropriate experimental control and troubleshooting tips learned through extensive experience with this model are provided. The surgical procedure itself takes ∼30 min to complete, with ∼2 h of perioperative care, and tissue collection is generally performed 4-12 weeks postoperatively. Several analytical techniques are presented, which evaluate the cellular and extracellular matrix components, functionality and mineralization, including histological, mechanical and radiographic methods.
Conflict of interest statement
Competing Interest Statement
The authors declare no competing financial interests.
Figures
Figure 1
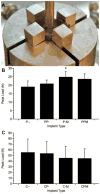
Use of the push-out jig. (A) Photograph of push-out jig. The peak push-out load of the specimens (n = 6) with implanted (B) polymeric and (C) ceramic scaffolds with or without platelet rich plasma and/or bone marrow derived mononuclear cells. Implant type abbreviations denote the scaffold material (polymer, P or ceramic, C) followed by the presence or absence of platelet rich plasma (- or P, respectively) and the presence or absence of mononuclear cells (or M, respectively). * indicates significant difference from material control (p <0.05). Adapted with permission.
Figure 2
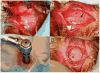
Creation of the defect. The bone is exposed and the defect created by incision and retraction of the (A) skin and (B) periosteum (shown with white arrowheads) overlying the calvarium. Note the clear line of the sagittal suture in the bone of the calvarium indicated with a black arrow. A trephine with 8 mm diameter is used to cut the calvarial bone (C) resulting in a scored calvarium (D). * indicates the anterior side of the cranium.
Figure 3
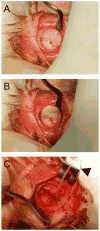
Use of the elevator. The elevator is used to gently lift the bone from the dura, first by (A) lifting the edge then (B) running the elevator under the surface to free any adherent dura. The exposed dura and brain beneath the defect are shown in (C). Additionally, a 4-0 Monocryl suture is seen loosely threaded through the periosteum indicated by the black arrowhead in (C). This technique of beginning the suture before trephination allows for easy apposition of the periosteal layer as it is very thin and delicate.
Figure 4
Scoring guide for extent of bony bridging and union. The gray areas in the circles represent mineralized tissue formed within the defect, which can be used for planar radiographs or microCT datasets. Reprinted with permission.
Figure 5
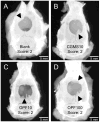
Representative planar radiographs of defects at 12 weeks from a study looking at release of plasmid DNA encoding BMP-2 from a hydrogel (OPF) with gelatin microparticles (CGMS). The images show specimens implanted with (A) OPF and CGMS, (B) OPF and 10 μg of pDNA in the CGMS phase, (C) CGMS and 10 μg of pDNA in the OPF phase and (D) CGMS and 100 μg of pDNA in OPF phase. Arrowheads indicate the areas of bone growth into the defect.
Figure 6
Histological sections cut coronally through the defect after 12 weeks of implantation. Ceramic (A–B) and polymeric (C–E) scaffolds were used in combination with platelet rich plasma (PRP) and/or bone marrow derived mononuclear cells (bmMNCs). The sections are stained with (A, D) Goldner’s trichrome, (B, E) hematoxylin and eosin and (C) von Kossa/van Gieson and show representative sections from the (A) ceramic scaffold with PRP group, (B) the ceramic scaffold with PRP and bmMNCs, (C) the polymeric scaffold with bmMNCs, (D) the polymeric scaffold with PRP and bmMNCs and (E) the polymeric scaffold alone. Arrows indicate mineralized tissue and arrowheads indicate non-mineralized osteoid. Scale bars for the full size images on the left represent 1 mm, while scale bars for the higher magnifications on the right represent 100 μm. Adapted with permission.
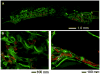
Figure 7
Fluorescent image of a coronally oriented histological section showing temporal fluorochrome labeled mineral deposition. This study implanted titanium meshes seeded with bone marrow derived marrow stromal cells transfected with an adenoviral vector of BMP-2. Red fluorescence is due to alizarin complexone injected at 1 week and green fluorescence is due to calcein injected at 3 weeks postoperatively. Insets (B) and (C) show high magnification (10X) images of the section indicated by the white boxes in the image of the full defect (A) at low magnification (2.5X). Reprinted with permission.
Similar articles
- An Ovine Model of In Vivo Bioreactor-Based Bone Generation.
Watson E, Tatara AM, van den Beucken JJJP, Jansen JA, Wong ME, Mikos AG. Watson E, et al. Tissue Eng Part C Methods. 2020 Jul;26(7):384-396. doi: 10.1089/ten.TEC.2020.0125. Epub 2020 Jul 7. Tissue Eng Part C Methods. 2020. PMID: 32536266 Free PMC article. - Evaluation of an Engineered Hybrid Matrix for Bone Regeneration via Endochondral Ossification.
Mikael PE, Golebiowska AA, Xin X, Rowe DW, Nukavarapu SP. Mikael PE, et al. Ann Biomed Eng. 2020 Mar;48(3):992-1005. doi: 10.1007/s10439-019-02279-0. Epub 2019 Apr 29. Ann Biomed Eng. 2020. PMID: 31037444 Free PMC article. - Topographically Defined, Biodegradable Nanopatterned Patches to Regulate Cell Fate and Acceleration of Bone Regeneration.
Lee MS, Lee DH, Jeon J, Oh SH, Yang HS. Lee MS, et al. ACS Appl Mater Interfaces. 2018 Nov 14;10(45):38780-38790. doi: 10.1021/acsami.8b14745. Epub 2018 Oct 31. ACS Appl Mater Interfaces. 2018. PMID: 30360116 - Rodent models in bone-related research: the relevance of calvarial defects in the assessment of bone regeneration strategies.
Gomes PS, Fernandes MH. Gomes PS, et al. Lab Anim. 2011 Jan;45(1):14-24. doi: 10.1258/la.2010.010085. Epub 2010 Dec 14. Lab Anim. 2011. PMID: 21156759 Review. - Cell Cotransplantation Strategies for Vascularized Craniofacial Bone Tissue Engineering: A Systematic Review and Meta-Analysis of Preclinical In Vivo Studies.
Shanbhag S, Pandis N, Mustafa K, Nyengaard JR, Stavropoulos A. Shanbhag S, et al. Tissue Eng Part B Rev. 2017 Apr;23(2):101-117. doi: 10.1089/ten.TEB.2016.0283. Epub 2016 Nov 1. Tissue Eng Part B Rev. 2017. PMID: 27733094 Review.
Cited by
- The Axolotl Fibula as a Model for the Induction of Regeneration across Large Segment Defects in Long Bones of the Extremities.
Chen X, Song F, Jhamb D, Li J, Bottino MC, Palakal MJ, Stocum DL. Chen X, et al. PLoS One. 2015 Jun 22;10(6):e0130819. doi: 10.1371/journal.pone.0130819. eCollection 2015. PLoS One. 2015. PMID: 26098852 Free PMC article. - Evaluation of Biocomposite Cements for Bone Defect Repair in Rat Models.
Ardelean AI, Mârza SM, Marica R, Dragomir MF, Rusu-Moldovan AO, Moldovan M, Pașca PM, Oana L. Ardelean AI, et al. Life (Basel). 2024 Aug 30;14(9):1097. doi: 10.3390/life14091097. Life (Basel). 2024. PMID: 39337881 Free PMC article. - Trends in bioactivity: inducing and detecting mineralization of regenerative polymeric scaffolds.
Nitschke BM, Beltran FO, Hahn MS, Grunlan MA. Nitschke BM, et al. J Mater Chem B. 2024 Mar 13;12(11):2720-2736. doi: 10.1039/d3tb02674d. J Mater Chem B. 2024. PMID: 38410921 Free PMC article. Review. - Comparison of a Thiolated Demineralized Bone Matrix Hydrogel to a Clinical Product Control for Regeneration of Large Sheep Cranial Defects.
Townsend JM, Kiyotake EA, Easley J, Seim HB 3rd, Stewart HL, Fung KM, Detamore MS. Townsend JM, et al. Materialia (Oxf). 2023 Mar;27:101690. doi: 10.1016/j.mtla.2023.101690. Epub 2023 Jan 17. Materialia (Oxf). 2023. PMID: 36743831 Free PMC article. - Comprehensive In Vitro Testing of Calcium Phosphate-Based Bioceramics with Orthopedic and Dentistry Applications.
Albulescu R, Popa AC, Enciu AM, Albulescu L, Dudau M, Popescu ID, Mihai S, Codrici E, Pop S, Lupu AR, Stan GE, Manda G, Tanase C. Albulescu R, et al. Materials (Basel). 2019 Nov 10;12(22):3704. doi: 10.3390/ma12223704. Materials (Basel). 2019. PMID: 31717621 Free PMC article. Review.
References
- Giannoudis PV, Atkins R. Management of long-bone non-unions. Injury. 2007;38:S1–2. - PubMed
- Giannoudis PV, et al. Nonunion of the femoral diaphysis. The influence of reaming and non-steroidal anti-inflammatory drugs. J Bone Joint Surg Br. 2000;82:655–658. - PubMed
- Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986;205:299–308. - PubMed
- Cancedda R, Giannoni P, Mastrogiacomo M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomater. 2007;28:4240–4250. - PubMed
- Reichert JC, et al. Establishment of a preclinical ovine model for tibial segmental bone defect repair by applying bone tissue engineering strategies. Tissue Eng Part B Rev. 2010;16:93–104. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AR042639/AR/NIAMS NIH HHS/United States
- R21 AR056076/AR/NIAMS NIH HHS/United States
- R01 DE017441/DE/NIDCR NIH HHS/United States
- R01 AR42639/AR/NIAMS NIH HHS/United States
- R21 AR56076/AR/NIAMS NIH HHS/United States
- R01 DE015164/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources