Action of vitamin D and the receptor, VDRa, in calcium handling in zebrafish (Danio rerio) - PubMed (original) (raw)
Action of vitamin D and the receptor, VDRa, in calcium handling in zebrafish (Danio rerio)
Chia-Hao Lin et al. PLoS One. 2012.
Abstract
The purpose of the present study was to use zebrafish as a model to investigate how vitamin D and its receptors interact to control Ca(2+) uptake function. Low-Ca(2+) fresh water stimulated Ca(2+) influx and expressions of epithelial calcium channel (ecac), vitamin D-25-hydroxylase (cyp2r1), vitamin D receptor a (vdra), and vdrb in zebrafish. Exogenous vitamin D increased Ca(2+) influx and expressions of ecac and 25-hydroxyvitamin D(3)-24-hydroxylase (cyp24a1), but downregulated 1α-OHase (cyp27b1) with no effects on other Ca(2+) transporters. Morpholino oligonucleotide knockdown of VDRa, but not VDRb, was found as a consequence of calcium uptake inhibition by knockdown of ecac, and ossification of vertebrae is impaired. Taken together, vitamin D-VDRa signaling may stimulate Ca(2+) uptake by upregulating ECaC in zebrafish, thereby clarifying the Ca(2+)-handling function of only a VDR in teleosts. Zebrafish may be useful as a model to explore the function of vitamin D-VDR signaling in Ca(2+) homeostasis and the related physiological processes in vertebrates.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
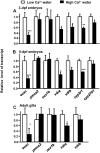
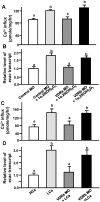
Figure 1. Effect of different Ca2+ concentrations on mRNA expressions of Ca2+-related genes.
(A) mRNA expressions in 3-d post-fertilization (dpf) zebrafish embryos. (B) mRNA expressions in 5-dpf zebrafish embryos. (C) mRNA expressions in gills of adult zebrafish. mRNA expressions were analyzed by a qPCR, and values were normalized to β-actin. Values are the mean ± SD (n = 4∼6). Student’s t-test, *p<0.05; **p<0.05; ***p<0.001.
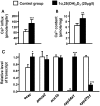
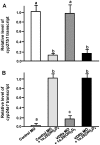
Figure 2. Effects of exogenous 1α,25(OH)2D3 (20 µg/l) in 3-d post-fertilization (dpf) zebrafish embryos.
Ca2+ influx (A), Ca2+ content (B), mRNA expressions in 3-dpf zebrafish embryos (C). mRNA expressions were analyzed by a qPCR, and values were normalized to β-actin. Values are the mean ± SD (n = 7∼10). Student’s t-test, *p<0.05; **p<0.01, ***p<0.001.
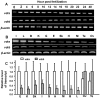
Figure 3. Alignment and phylogenetic analysis of vitamin D receptors (VDRs).
(A) Alignment of amino-acid sequences of zebrafish (z)VDRa and VDRb. (B) Phylogenetic analysis of vertebrate VDRs. The consensus line denotes a consensus (asterisk), similarity (: or.), or difference (-) between zVDRa and zVDRb. Bold letters indicate the DNA-binding domain, and underlined letters indicate the ligand-binding domain. Phylogenetic analyses were conducted using MEGA5. The phylogenetic analyses were inferred using Neighbor-joining trees and were bootstrapped (600 pseudosamples) to assess the robustness. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test is shown next to the branches. The unit of the scale bar is the number of amino-acid substitutions per site.
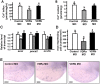
Figure 4. Zebrafish vdra and vdrb expression profiles.
mRNA expression patterns in developing stages (A) and various tissues (B) were determined by an RT-PCR using β-actin as the internal control. (C) vdra and vdrb mRNA expressions in different tissues of adult zebrafish were analyzed by a qPCR using β-actin as the internal control. Values are the mean ± SD (n = 3). E, eye; K, kidney; Sk, skin; I, intestine; G, gill; H, heart; M, muscle; B, brain; L, liver; Ov, ovary; Te, testis.
Figure 5. Effects of vitamin D receptor (VDR)a and VDRb morpholino oligonucleotides (MOs) in 3-d post-fertilization (dpf) zebrafish embryos.
Ca2+ influx (A), Ca2+ content (B), mRNA expression (C), density of _ecac_-expressing cells (D), and ecac signals (E). mRNA expressions were analyzed by a qPCR using β-actin as the internal control. Different letters indicate a significant difference (p<0.05) using a one-way ANOVA followed by Tukey’s multiple-comparison test. Values are the mean ± SD (n = 6 or 7). Scale bar:100 µm.
Figure 6. Effects of vitamin D receptor (VDR)a and VDRb morpholino oligonucleotides (MOs) on zebrafish embryos with 1α,25(OH)2D3 (20 µg/l) or low Ca2+ (0.02 mM; LCa) treatment.
Ca2+ influx (A, C) and ecac mRNA expression (B, D). mRNA expression of ecac was analyzed by a qPCR using β-actin as the internal control. Different letters indicate a significant difference (p<0.05) using one-way ANOVA followed by Tukey’s multiple-comparison test. Values are the mean ± SD. (n = 6∼8). High Ca2+ (HCa): 2.00 mM.
Figure 7. Effects of vitamin D receptor (VDR)a and VDRb morpholino oligonucleotides (MOs) on cyp27b1 and cyp24a1 mRNA expressions in 3-d post-fertilization (dpf) zebrafish embryos treated with 1α,25(OH)2D3 (20 µg/l).
(A) cyp27b1 mRNA expression. (B) cyp24a1 mRNA expression. mRNA expressions were analyzed by a qPCR using β-actin as the internal control. Different letters indicate a significant difference (p<0.05) using one-way ANOVA followed by Tukey’s multiple-comparison test. Values are the mean ± SD. (n = 5–6).
Similar articles
- Reverse effect of mammalian hypocalcemic cortisol in fish: cortisol stimulates Ca2+ uptake via glucocorticoid receptor-mediated vitamin D3 metabolism.
Lin CH, Tsai IL, Su CH, Tseng DY, Hwang PP. Lin CH, et al. PLoS One. 2011;6(8):e23689. doi: 10.1371/journal.pone.0023689. Epub 2011 Aug 24. PLoS One. 2011. PMID: 21887296 Free PMC article. - Inhibition of calcium uptake during hypoxia in developing zebrafish is mediated by hypoxia-inducible factor.
Kwong RW, Kumai Y, Tzaneva V, Azzi E, Hochhold N, Robertson C, Pelster B, Perry SF. Kwong RW, et al. J Exp Biol. 2016 Dec 15;219(Pt 24):3988-3995. doi: 10.1242/jeb.148700. Epub 2016 Oct 24. J Exp Biol. 2016. PMID: 27802147 - Temporal changes in tissue 1α,25-dihydroxyvitamin D3, vitamin D receptor target genes, and calcium and PTH levels after 1,25(OH)2D3 treatment in mice.
Chow EC, Quach HP, Vieth R, Pang KS. Chow EC, et al. Am J Physiol Endocrinol Metab. 2013 May 1;304(9):E977-89. doi: 10.1152/ajpendo.00489.2012. Epub 2013 Mar 12. Am J Physiol Endocrinol Metab. 2013. PMID: 23482451 - The Control of Calcium Metabolism in Zebrafish (Danio rerio).
Lin CH, Hwang PP. Lin CH, et al. Int J Mol Sci. 2016 Oct 26;17(11):1783. doi: 10.3390/ijms17111783. Int J Mol Sci. 2016. PMID: 27792163 Free PMC article. Review. - Intestinal calcium absorption: Molecular vitamin D mediated mechanisms.
Bouillon R, Van Cromphaut S, Carmeliet G. Bouillon R, et al. J Cell Biochem. 2003 Feb 1;88(2):332-9. doi: 10.1002/jcb.10360. J Cell Biochem. 2003. PMID: 12520535 Review.
Cited by
- Molecular cloning, functional characterization, and evolutionary analysis of vitamin D receptors isolated from basal vertebrates.
Kollitz EM, Zhang G, Hawkins MB, Whitfield GK, Reif DM, Kullman SW. Kollitz EM, et al. PLoS One. 2015 Apr 9;10(4):e0122853. doi: 10.1371/journal.pone.0122853. eCollection 2015. PLoS One. 2015. PMID: 25855982 Free PMC article. - Development of a Whole Organism Platform for Phenotype-Based Analysis of IGF1R-PI3K-Akt-Tor Action.
Liu C, Dai W, Bai Y, Chi C, Xin Y, He G, Mai K, Duan C. Liu C, et al. Sci Rep. 2017 May 17;7(1):1994. doi: 10.1038/s41598-017-01687-3. Sci Rep. 2017. PMID: 28515443 Free PMC article. - Vitamin D: calcium and bone homeostasis during evolution.
Bouillon R, Suda T. Bouillon R, et al. Bonekey Rep. 2014 Jan 8;3:480. doi: 10.1038/bonekey.2013.214. eCollection 2014 Jan 8. Bonekey Rep. 2014. PMID: 24466411 Free PMC article. Review. - Emerging role of vitamin D3 in alleviating intestinal structure injury caused by Aeromonas hydrophila in grass carp (Ctenopharyngodon idella).
Zhang Y, Zhou XQ, Jiang WD, Wu P, Liu Y, Ren HM, Zhang L, Mi HF, Tang L, Zhong CB, Feng L. Zhang Y, et al. Anim Nutr. 2023 Dec 20;16:202-217. doi: 10.1016/j.aninu.2023.07.010. eCollection 2024 Mar. Anim Nutr. 2023. PMID: 38362511 Free PMC article. - The role of cAMP-mediated intracellular signaling in regulating Na+ uptake in zebrafish larvae.
Kumai Y, Kwong RW, Perry SF. Kumai Y, et al. Am J Physiol Regul Integr Comp Physiol. 2014 Jan 1;306(1):R51-60. doi: 10.1152/ajpregu.00317.2013. Epub 2013 Nov 20. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 24259461 Free PMC article.
References
- Patschan D, Loddenkemper K, Buttgereit F (2001) Molecular mechanisms of glucocorticoid-induced osteoporosis. Bone 29: 498–505. - PubMed
- Vanoevelen J, Janssens A, Huitema LF, Hammond CL, Metz JR, et al. (2011) Trpv5/6 is vital for epithelial calcium uptake and bone formation. FASEB J 25(9): 3197–207. - PubMed
- Hwang PP, Perry SF (2010) Ionic and acid-base regulation. In: Pery SF, Ekker M, Farrell AP, Brauner CJ (ed) Zebrafish: A Fish Physiology, ed. vol.29. Academic Press, New York, NY, USA. 311–344.
- Hwang PP, Lee TH, Lin LY (2011) Ion Regulation in Fish Gills: Recent Progress in the Cellular and Molecular Mechanisms. Am J Physiol Regul Integr Comp Physiol 301: R28–R47. - PubMed
- Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85: 97–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This study was financially supported by the grant to P.P.H. from the National Science Council, Taiwan, R.O.C. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous