Effect of phenolic acids of microbial origin on production of reactive oxygen species in mitochondria and neutrophils - PubMed (original) (raw)
Effect of phenolic acids of microbial origin on production of reactive oxygen species in mitochondria and neutrophils
Natalia Beloborodova et al. J Biomed Sci. 2012.
Abstract
Background: Several low-molecular-weight phenolic acids are present in the blood of septic patients at high levels. The microbial origin of the most of phenolic acids in the human body was shown previously, but pathophysiological role of the phenolic acids is not clear. Sepsis is associated with the excessive production of reactive oxygen species (ROS) in both the circulation and the affected organs. In this work the influence of phenolic acids on ROS production in mitochondria and neutrophils was investigated.
Methods: ROS production in mitochondria and neutrophils was determined by MCLA- and luminol-dependent chemiluminescence. The rate of oxygen consumption by mitochondria was determined polarographically. The difference of electric potentials on the inner mitochondrial membrane was registered using a TPP+-selective electrode. The formation of phenolic metabolites in monocultures by the members of the main groups of the anaerobic human microflora and aerobic pathogenic bacteria was investigated by the method of gas chromatography-mass spectrometry.
Results: All phenolic acids had impact on mitochondria and neutrophils, the main producers of ROS in tissues and circulation. Phenolic acids (benzoic and cinnamic acids) producing the pro-oxidant effect on mitochondria inhibited ROS formation in neutrophils. Their effect on mitochondria was abolished by dithiothreitol (DTT). Phenyllactate and p-hydroxyphenyllactate decreased ROS production in both mitochondria and neutrophils. Bifidobacteria and lactobacilli produced in vitro considerable amounts of phenyllactic and p-hydroxyphenyllactic acids, Clostridia s. produced great quantities of phenylpropionic and p-hydroxyphenylpropionic acids, p-hydroxyphenylacetic acid was produced by Pseudomonas aeruginosa and Acinetobacter baumanii; and benzoic acid, by Serratia marcescens.
Conclusions: The most potent activators of ROS production in mitochondria are phenolic acids whose effect is mediated via the interaction with thiol groups. Among these are benzoic and cinnamic acids. Some phenolic acids, in particular phenyllactate and p-hydroxyphenyllactate, which decrease ROS production in mitochondria and neutrophils, can play a role of natural antioxidants. The results indicate that low-molecular weight phenolic acids of microbial origin participate in the regulation of the ROS production in both the circulation and tissues, thereby affecting the level of oxidative stress in sepsis.
Figures
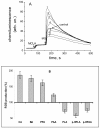
Figure 1
Effect of phenolic acids on ROS production in mitochondria. A- Effect of phenolic acids on chemiluminescence of MCLA in mitochondria in the presence of redox-cycler menadione (MQ): 1, cinnamic; 2- benzoic, 3- phenylpropionic, 4- phenylacetic, 5- control, 6- p-hydroxyphenylpropionic, 7- phenyllactic; the concentrations of phenolic acids 100 μM, menadione 25 μM, MCLA 40 μM, pyruvate 4 mM, and rotenone 2.5 μM. B- Comparison of the effect of phenolic acids: cinnamic (CA), benzoic (BA), phenylpropionic (PPA), phenylacetic (PAA), _p_-hydroxyphenylpropionic (_p_-HPPA), phenyllactic (PLA), _p_-hydroxyphenyllactic (_p_-HPLA) at a concentration of 100 μM on menadione-activated ROS production. Data are expressed as the means ± SEM of five independent determinations.
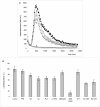
Figure 2
Effect of phenolic acids of microbial origin on NAD- dependent respiration of mitochondria. The inhibition of NAD- dependent respiration of mitochondria by cinnamic acid was taken to be 100%. The concentration of phenolic acids was 100 μM. Data are expressed as the means ± SEM of five independent determinations.
Figure 3
Effect of cinnamic and benzoic acids on mitochondrial membrane potential. A- Effect of cinnamic acid (CA) on ΔΨm upon the oxidation of pyruvate and its elimination by succinate; B – Removal of the effects of cinnamic (CA) and benzoic (BA) acids by DTT. The concentrations of phenolic acids were 200 μM, DTT 2 mM, pyruvate 4 mM, and succinate 4 mM.
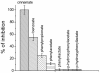
Figure 4
Effect of phenolic acids of microbial origin and thiol reagents on ROS production in neutrophils. A – chemiluminescence of luminol in PMA-activated neutrophils, 1- control, 2- benzoic acid, 3- cinnamic acid, 4- NEM, 25 μМ; B- effect of the phenolic acids (100 μМ) and thiols reagents (DTT, 1 mM and NEM, 5 and 25 μМ) on ROS production in neutrophils.
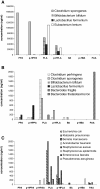
Figure 5
Species-specific production of phenolic acids by anaerobic human microbiota and pathogenic facultative aerobes. A- abundant phenolic acids (ng/ml) produced by anaerobic human microbiota; B – minor phenolic acids (ng/ml) produced by the same microbiota; C - phenolic acids (ng/ml) produced by pathogenic facultative aerobes.
Similar articles
- Toxic effects of microbial phenolic acids on the functions of mitochondria.
Fedotcheva NI, Kazakov RE, Kondrashova MN, Beloborodova NV. Fedotcheva NI, et al. Toxicol Lett. 2008 Aug 28;180(3):182-8. doi: 10.1016/j.toxlet.2008.06.861. Epub 2008 Jun 26. Toxicol Lett. 2008. PMID: 18634861 - Inhibition of chemiluminescence in human PMNs by monocyclic phenolic acids and flavonoids.
Limasset B, Ojasoo T, le Doucen C, Doré JC. Limasset B, et al. Planta Med. 1999 Feb;65(1):23-9. doi: 10.1055/s-1999-13956. Planta Med. 1999. PMID: 10083840 - Oxidative Stress and the Aging Brain: From Theory to Prevention.
Gemma C, Vila J, Bachstetter A, Bickford PC. Gemma C, et al. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 15. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 15. PMID: 21204345 Free Books & Documents. Review.
Cited by
- The impact of bed rest on human skeletal muscle metabolism.
Eggelbusch M, Charlton BT, Bosutti A, Ganse B, Giakoumaki I, Grootemaat AE, Hendrickse PW, Jaspers Y, Kemp S, Kerkhoff TJ, Noort W, van Weeghel M, van der Wel NN, Wesseling JR, Frings-Meuthen P, Rittweger J, Mulder ER, Jaspers RT, Degens H, Wüst RCI. Eggelbusch M, et al. Cell Rep Med. 2024 Jan 16;5(1):101372. doi: 10.1016/j.xcrm.2023.101372. Cell Rep Med. 2024. PMID: 38232697 Free PMC article. - Metabolic signatures of germination triggered by kinetin in Medicago truncatula.
Araújo S, Pagano A, Dondi D, Lazzaroni S, Pinela E, Macovei A, Balestrazzi A. Araújo S, et al. Sci Rep. 2019 Jul 18;9(1):10466. doi: 10.1038/s41598-019-46866-6. Sci Rep. 2019. PMID: 31320688 Free PMC article. - Exercise training modifies xenometabolites in gut and circulation of lean and obese adults.
Kasperek MC, Mailing L, Piccolo BD, Moody B, Lan R, Gao X, Hernandez-Saavedra D, Woods JA, Adams SH, Allen JM. Kasperek MC, et al. Physiol Rep. 2023 Mar;11(6):e15638. doi: 10.14814/phy2.15638. Physiol Rep. 2023. PMID: 36945966 Free PMC article. - Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes.
Masumoto S, Terao A, Yamamoto Y, Mukai T, Miura T, Shoji T. Masumoto S, et al. Sci Rep. 2016 Aug 10;6:31208. doi: 10.1038/srep31208. Sci Rep. 2016. PMID: 27506289 Free PMC article. - Fecal microbiome and metabolome of infants fed bovine MFGM supplemented formula or standard formula with breast-fed infants as reference: a randomized controlled trial.
He X, Parenti M, Grip T, Lönnerdal B, Timby N, Domellöf M, Hernell O, Slupsky CM. He X, et al. Sci Rep. 2019 Aug 12;9(1):11589. doi: 10.1038/s41598-019-47953-4. Sci Rep. 2019. PMID: 31406230 Free PMC article. Clinical Trial.
References
- Víctor VM, Espulgues JV, Hernández-Mijares A, Rocha M. Oxidative stress and mitochondrial dysfunction in sepsis: a potential therapy with mitochondria-targeted antioxidants. Infect Disord Drug Targets. 2009;9:376–389. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources