Serum microRNA-155 as a potential biomarker to track disease in breast cancer - PubMed (original) (raw)
Controlled Clinical Trial
Serum microRNA-155 as a potential biomarker to track disease in breast cancer
Yu Sun et al. PLoS One. 2012.
Abstract
Background: One major impediment to improving the management of breast cancer is the current lack of tumor marker with sufficient sensitivity and specificity. A growing body of evidence implicates the diagnostic potential of circulating miRNAs in cancer detection. MiR-155 plays an important role in the pathogenesis of breast cancer. However, the level of circulating miR-155 and its clinical relevance are not well established. The objective of the current study was to learn more about serum miR-155 in patients with breast cancer.
Methodology/principal findings: Using quantitative reverse transcription polymerase chain reaction (RT-qPCR), we demonstrated that serum miR-155 had significant increased levels in breast cancer patients (n = 103) compared with healthy subjects (n = 55) (p<0.001), which had a mean fold change of 2.94. Receiver operating characteristic (ROC) analysis revealed that miR-155 had considerable diagnostic accuracy, yielding an ROC-AUC (the areas under the ROC curve) of 0.801 (sensitivity 65.0%, specificity 81.8%). In addition, sera from a subset of breast cancer patients (n = 29) were collected after surgery and after four cycles of chemotherapy to evaluate the effects of clinical treatment on serum levels of candidate miRNAs. Surprisingly, a decreased level of serum miR-155 was found; whereas the concentrations of carbohydrate antigen 15-3 (CA15-3), carcinoembryonic antigen (CEA) and tissue polypeptide specific antigen (TPS) did not show this trend. Our results revealed that 79% patients showed response or stable disease after therapy had declined levels of serum miR-155.
Conclusions/significance: Our results suggest that serum miR-155 is a potential biomarker to discriminate breast cancer patients from healthy subjects. For the first time, we demonstrated a declined trend of miR-155 after surgery and chemotherapy, which raises the possibility to use it as an indicator for treatment response.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
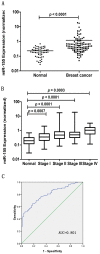
Figure 1. Serum miR-155 levels in normal controls (n = 55) and breast cancer patients (n = 103).
The relative expression level of miR-155 was normalized to spiked-in cel-mir-39. The line represents the median value. Statistically significant difference was determined using Mann-Whitney tests. The result revealed a higher level of miR-155 in breast cancer patients (p<0.001) (A). Box plot of serum miR-155 expression levels across stages. The boxes indicate the 25th and 75th percentiles and the bold lines represent the median values. The relative expression levels of target miRNA were normalized to spiked-in cel-mir-39. Statistically significant differences were determined using Mann-Whitney tests or Kruskal-Wallis tests (B). Receiver operating characteristics (ROC) curve analysis for the diagnostic value of miR-155. The AUC (the areas under the ROC curve) was 0.801 (95% CI: 0.734 to 0.868) (C).
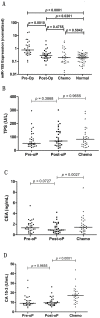
Figure 2. Changes in serum miR-155 and CA 15-3, CEA, TPS levels before (Pre-oP) and after surgery (Post-oP), and after chemotherapy (Chemo).
Sera (n = 29) were collected from patients who underwent curative resection and adjuvant chemotherapy. The levels of miR-155 were significantly reduced after surgery (p = 0.0002), reaching levels comparable with healthy subjects (p = 0.5042) (A). The levels of TPS remained unchanged (_p_>0.05) (B). The levels of CA 15-3 and CEA underwent a significant elevation after chemotherapy (p = 0.0027 for CEA, p<0.0001 for CA 15-3) (C–D).
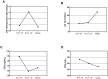
Figure 3. Changes in serum levels of miR-155, CA 15-3, CEA, and TPS in the patient who experienced relapse before (Pre-oP) and after surgery (Post-oP), and after chemotherapy (Chemo).
The level of miR-155 underwent a 250% increase after surgery, and decreased to the preoperative level after chemotherapy (A). In light of a change in the serum marker levels ≥25% was regarded as a significant alteration, the level of CA 15-3 did not change after surgery but significantly elevated after chemotherapy (B); the level of CEA decreased after surgery (C); the level of TPS did not change (D).
Similar articles
- Assessment of serum microRNA-21 and miRNA-205 as diagnostic markers for stage I and II breast cancer in Indian population.
Rama K, Bitla AR, Hulikal N, Yootla M, Yadagiri LA, Asha T, Manickavasagam M, Srinivasa Rao P. Rama K, et al. Indian J Cancer. 2024 Apr 1;61(2):290-298. doi: 10.4103/ijc.IJC_187_20. Epub 2023 Jul 17. Indian J Cancer. 2024. PMID: 38090957 - Thioredoxin 1 as a serum marker for breast cancer and its use in combination with CEA or CA15-3 for improving the sensitivity of breast cancer diagnoses.
Park BJ, Cha MK, Kim IH. Park BJ, et al. BMC Res Notes. 2014 Jan 6;7:7. doi: 10.1186/1756-0500-7-7. BMC Res Notes. 2014. PMID: 24393391 Free PMC article. Clinical Trial. - Diagnostic Value of Serum miR-182, miR-183, miR-210, and miR-126 Levels in Patients with Early-Stage Non-Small Cell Lung Cancer.
Zhu W, Zhou K, Zha Y, Chen D, He J, Ma H, Liu X, Le H, Zhang Y. Zhu W, et al. PLoS One. 2016 Apr 19;11(4):e0153046. doi: 10.1371/journal.pone.0153046. eCollection 2016. PLoS One. 2016. PMID: 27093275 Free PMC article. - Plasma mammaglobin messenger RNA in breast cancer patients as an addition to serum tumor.
El-Attar NI, Gaefar HA. El-Attar NI, et al. Egypt J Immunol. 2007;14(2):111-21. Egypt J Immunol. 2007. PMID: 20306663 - Exploring research progress in studying serum exosomal miRNA-21 as a molecular diagnostic marker for breast cancer.
Li H, Tie XJ. Li H, et al. Clin Transl Oncol. 2024 Sep;26(9):2166-2171. doi: 10.1007/s12094-024-03454-z. Epub 2024 Apr 11. Clin Transl Oncol. 2024. PMID: 38602645 Review.
Cited by
- Combining miRNA and mRNA Expression Profiles in Wilms Tumor Subtypes.
Ludwig N, Werner TV, Backes C, Trampert P, Gessler M, Keller A, Lenhof HP, Graf N, Meese E. Ludwig N, et al. Int J Mol Sci. 2016 Mar 30;17(4):475. doi: 10.3390/ijms17040475. Int J Mol Sci. 2016. PMID: 27043538 Free PMC article. - lnc-3215 Suppression Leads to Calcium Overload in Selenium Deficiency-Induced Chicken Heart Lesion via the lnc-3215-miR-1594-TNN2 Pathway.
Yang J, Gong Y, Cai J, Liu Q, Zhang Z. Yang J, et al. Mol Ther Nucleic Acids. 2019 Dec 6;18:1-15. doi: 10.1016/j.omtn.2019.08.003. Epub 2019 Aug 12. Mol Ther Nucleic Acids. 2019. PMID: 31479920 Free PMC article. - Breast cancer tumor microenvironment affects Treg/IL-17-producing Treg/Th17 cell axis: Molecular and therapeutic perspectives.
Seif F, Torki Z, Zalpoor H, Habibi M, Pornour M. Seif F, et al. Mol Ther Oncolytics. 2023 Jan 11;28:132-157. doi: 10.1016/j.omto.2023.01.001. eCollection 2023 Mar 16. Mol Ther Oncolytics. 2023. PMID: 36816749 Free PMC article. Review. - Increased circulating microRNA-155 as a potential biomarker for breast cancer screening: a meta-analysis.
Wang F, Hou J, Jin W, Li J, Yue Y, Jin H, Wang X. Wang F, et al. Molecules. 2014 May 16;19(5):6282-93. doi: 10.3390/molecules19056282. Molecules. 2014. PMID: 24840899 Free PMC article. - Circulating microRNAs in breast cancer: novel diagnostic and prognostic biomarkers.
Hamam R, Hamam D, Alsaleh KA, Kassem M, Zaher W, Alfayez M, Aldahmash A, Alajez NM. Hamam R, et al. Cell Death Dis. 2017 Sep 7;8(9):e3045. doi: 10.1038/cddis.2017.440. Cell Death Dis. 2017. PMID: 28880270 Free PMC article. Review.
References
- Anonymous (1996) Clinical practice guidelines for the use of tumor markers in breast and colorectal cancer. Adopted on May 17, 1996 by the American Society of Clinical Oncology. J Clin Oncol 14: 2843–2877. - PubMed
- Guadagni F, Ferroni P, Carlini S, Mariotti S, Spila A, et al. (2001) A re-evaluation of carcinoembryonic antigen (CEA) as a serum marker for breast cancer: a prospective longitudinal study. Clin Cancer Res 7: 2357–2362. - PubMed
- Duffy MJ, Evoy D, McDermott EW (2010) CA 15–3: uses and limitation as a biomarker for breast cancer. Clin Chim Acta 411: 1869–1874. - PubMed
- Harris L, Fritsche H, Menel R, Norton L, Ravdin P, et al. (2007) American Society of Clinical Oncology 2007 Update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25: 5287–5312. - PubMed
- Sturgeon CM, Duffy MJ, Stenman UK, Lilja H, Brünner N, et al. (2008) National Academy of Clinical Biochemistry Laboratory Medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast and ovarian cancers. Clin Chem 54: e11–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was supported by a grant from the National High Technology Reseach and Development Program of China (863 Program) (No. 2011AA02A116). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical