Inhibition of cellular autophagy deranges dengue virion maturation - PubMed (original) (raw)
Inhibition of cellular autophagy deranges dengue virion maturation
Roberto Mateo et al. J Virol. 2013 Feb.
Abstract
Autophagy is an important component of the innate immune response, directly destroying many intracellular pathogens. However, some pathogens, including several RNA viruses, subvert the autophagy pathway, or components of the pathway, to facilitate their replication. In the present study, the effect of inhibiting autophagy on the growth of dengue virus was tested using a novel inhibitor, spautin-1 (specific and potent autophagy inhibitor 1). Inhibition of autophagy by spautin-1 generated heat-sensitive, noninfectious dengue virus particles, revealing a large effect of components of the autophagy pathway on viral maturation. A smaller effect on viral RNA accumulation was also observed. Conversely, stimulation of autophagy resulted in increased viral titers and pathogenicity in the mouse. We conclude that the presence of functional autophagy components facilitates viral RNA replication and, more importantly, is required for infectious dengue virus production. Pharmacological inhibition of host processes is an attractive antiviral strategy to avoid selection of treatment-resistant variants, and inhibitors of autophagy may prove to be valuable therapeutics against dengue virus infection and pathogenesis.
Figures
Fig 1
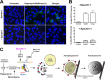
Effect of spautin-1 on the formation of rapamycin-induced autophagosomes and dengue virus-induced LC3-containing membranous structures. (A) Huh7.A.1-GFP-LC3 cells were untreated, treated with 0.5 μM rapamycin and 50 nM bafilomycin, or infected with dengue virus at an MOI of 0.1 in either the presence (+) or absence (−) of 10 μM spautin-1. Images were taken after a 24-hour incubation period. Nuclei were visualized by DAPI staining. (B) The percentage of GFP-LC3-containing cells was calculated in 10 random fields. Shown are the averages ± standard errors of the mean (SEM). (C) Overview of the autophagy pathway with the drugs used in this study and their targets.
Fig 2
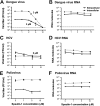
Effects of spautin-1 on dengue virus, HCV, and poliovirus titers and RNA. (A and B) BHK-21 cells were infected with dengue virus at an MOI of 0.1 FFU/cell and left untreated or treated with various spautin-1 concentrations for 24 h. Extracellular and intracellular titers were determined (A), and amounts of viral RNA were quantitated using qRT-PCR (B). (C and D) Huh7.5.1 cells were infected with HCV at an MOI of 0.1 FFU/cell and left untreated or treated with various spautin-1 concentrations for 72 h. Extracellular and intracellular virus titers (C) and viral RNA (D) were quantified. (E and F) HeLa cells were infected with poliovirus at an MOI of 0.1 PFU/cell and left untreated or treated with spautin-1 for 6 h. Virus titers (E) and viral RNA (F) amounts were determined. When spautin-1 was less than 10 μM, IC90 values are indicated for all three viruses. All data shown are averages ± SEM of three or four biological replicates.
Fig 3
Effect of autophagy stimulation and fatty acid supplementation upon 3-MA and spautin-1 treatment of BHK-21 cells on extracellular dengue virus yield. (A and B) BHK-21 cells were infected with dengue virus at an MOI of 0.1 FFU/cell and treated with increasing concentrations of rapamycin (A) and nicardipine (B) for 24 h. Supernatants were collected, and titers were determined on fresh BHK-21 monolayers. Experiments representative of several performed are shown. (C and D) BHK-21 cells were similarly infected with dengue virus at an MOI of 0.1 FFU/cell and left untreated or treated with 1.2 mM 3-MA (C) or 10 μM spautin-1 (D) in the presence or absence of oleic acid-BSA or BSA alone for 24 h. Extracellular titers and SEM were determined by focus-forming-unit assay. *, P < 0.05. A dashed line indicates the limit of detection of 7 FFU/ml for the titration assay.
Fig 4
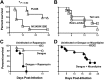
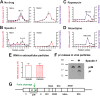
Pathogenicity of dengue virus in autophagy-stimulated AG129 mice. (A) AG129 mice were intravenously inoculated with 107 FFU/mouse of the wild-type dengue virus strain PL046 or virus containing the mutations N124D and K128E in the envelope protein (40), and a time course of lethality was determined. (B) Mice were intravenously inoculated with 107 FFU/mouse of dengue virus N124D/K128E via the tail vein or retro-orbitally. (C and D) Mice were retro-orbitally inoculated with 2 × 105 FFU of dengue virus N124D/K128E and treated with rapamycin (20 mg/kg) (C) or nicardipine (5 mg/kg) (D) by intraperitoneal injection every 12 h. Differences in pathogenicity between mice infected with dengue virus alone or dengue virus with rapamycin (C) or nicardipine (D) were statistically significant (P < 0.001 and P = 0.01, respectively).
Fig 5
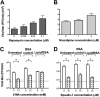
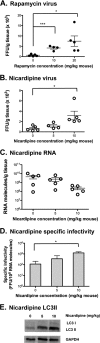
Effect of autophagy stimulation in AG129 mice on dengue virus and RNA accumulation. (A and B) Mice were retro-orbitally inoculated with 2 × 105 FFU/mouse of N124D/K128E dengue virus and treated with the indicated amounts of rapamycin or nicardipine every 12 h. Spleens were harvested, homogenized, and analyzed to determine the amounts of infectious virus under rapamycin (A) and nicardipine (B) stimulation. (C) Viral RNA was also determined in spleens of nicardipine-treated mice. Each point represents one individual mouse; the mean values ± SEM are shown. (D) Specific infectivity (FFU/108 molecules) in the spleen was determined for nicardipine-treated mice. (E) Gastrocnemius muscles were collected 48 h after infection, and protein homogenates were generated and blotted for the presence of LC3 using GAPDH as a loading control. A representative blot for nicardipine treatment is shown.***, P < 0.0005; *, P < 0.05.
Fig 6
Analysis of dengue virus particles formed upon spautin-1, rapamycin, and nicardipine treatment. (A to D) Supernatants from dengue virus-infected BHK-21 cells that were untreated (A) or had been treated with spautin-1 (B), rapamycin (C), or nicardipine (D) were loaded onto 5 to 50% sucrose gradients and subjected to velocity centrifugation. Fractions were collected and analyzed to determine the amounts of infectious virus and viral RNA. Supernatants from spautin-1-treated cells and untreated cells were heated for 1 h at 37°C and subjected to the same velocity sedimentation. RNA was extracted from the resulting fractions, and viral RNA was quantitated. For each panel, specific infectivities (infectious virus/105 RNA molecules) for each condition ± SEM are indicated over the virus and RNA peaks. (E and F) Extracellular viral particles produced upon spautin-1 treatment were pelleted. Viral RNA was extracted and quantitated by qRT-PCR (E), and particles were analyzed by Western blotting for detection of prM protein (F). (G) dengue virus genome showing the maturation cleavage in prM protein.
Fig 7
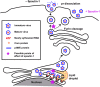
Model of spautin-1 action on the dengue virus life cycle. Viral RNA synthesis and formation of particles by budding into the ER are affected by spautin-1 treatment. Once immature, spiky viruses are formed in the ER lumen, they are transported via the secretory pathway through the Golgi network, where furin cleaves the structural prM protein, generating mature M protein and pr peptide, which remain associated with the virion until released in the extracellular milieu. This event produces viruses that are highly infectious. However, upon spautin-1 treatment, the pr peptide remains associated with the virions even after reaching the extracellular space, which renders them noninfectious.
Similar articles
- A Proline-Rich N-Terminal Region of the Dengue Virus NS3 Is Crucial for Infectious Particle Production.
Gebhard LG, Iglesias NG, Byk LA, Filomatori CV, De Maio FA, Gamarnik AV. Gebhard LG, et al. J Virol. 2016 May 12;90(11):5451-61. doi: 10.1128/JVI.00206-16. Print 2016 Jun 1. J Virol. 2016. PMID: 27009958 Free PMC article. - Regulation of autophagy, glucose uptake, and glycolysis under dengue virus infection.
Lee YR, Wu SY, Chen RY, Lin YS, Yeh TM, Liu HS. Lee YR, et al. Kaohsiung J Med Sci. 2020 Nov;36(11):911-919. doi: 10.1002/kjm2.12271. Epub 2020 Aug 12. Kaohsiung J Med Sci. 2020. PMID: 32783363 - Dengue Virus Inhibition of Autophagic Flux and Dependency of Viral Replication on Proteasomal Degradation of the Autophagy Receptor p62.
Metz P, Chiramel A, Chatel-Chaix L, Alvisi G, Bankhead P, Mora-Rodriguez R, Long G, Hamacher-Brady A, Brady NR, Bartenschlager R. Metz P, et al. J Virol. 2015 Aug;89(15):8026-41. doi: 10.1128/JVI.00787-15. J Virol. 2015. PMID: 26018155 Free PMC article. - Regulation of innate immune signaling pathways by autophagy in dengue virus infection.
Wan SW, Lee YR, Ho TS, Chang CP. Wan SW, et al. IUBMB Life. 2022 Feb;74(2):170-179. doi: 10.1002/iub.2554. Epub 2021 Sep 22. IUBMB Life. 2022. PMID: 34553486 Review. - The role of the unfolded protein response in dengue virus pathogenesis.
Perera N, Miller JL, Zitzmann N. Perera N, et al. Cell Microbiol. 2017 May;19(5). doi: 10.1111/cmi.12734. Epub 2017 Mar 8. Cell Microbiol. 2017. PMID: 28207988 Review.
Cited by
- Regulation of Autophagosome-Lysosome Fusion by Human Viral Infections.
Ke PY. Ke PY. Pathogens. 2024 Mar 20;13(3):266. doi: 10.3390/pathogens13030266. Pathogens. 2024. PMID: 38535609 Free PMC article. Review. - Novel Potent Autophagy Inhibitor Ka-003 Inhibits Dengue Virus Replication.
Limthongkul J, Akkarasereenon K, Yodweerapong T, Songthammawat P, Tong-Ngam P, Tubsuwan A, Kunkaew N, Kanjanasirirat P, Khumpanied T, Wannalo W, Ubol S, Borwornpinyo S, Ploypradith P, Ponpuak M. Limthongkul J, et al. Viruses. 2023 Sep 27;15(10):2012. doi: 10.3390/v15102012. Viruses. 2023. PMID: 37896789 Free PMC article. - Crosstalk between Autophagy and RLR Signaling.
Ke PY. Ke PY. Cells. 2023 Mar 21;12(6):956. doi: 10.3390/cells12060956. Cells. 2023. PMID: 36980296 Free PMC article. Review. - The role of autophagy in viral infections.
Chen T, Tu S, Ding L, Jin M, Chen H, Zhou H. Chen T, et al. J Biomed Sci. 2023 Jan 18;30(1):5. doi: 10.1186/s12929-023-00899-2. J Biomed Sci. 2023. PMID: 36653801 Free PMC article. Review. - Plasma interleukin-7 correlation with human immunodeficiency virus RNA and CD4+ T cell counts, and interleukin-5 with circulating hepatitis B virus DNA may have implications in viral control.
Vimali J, Yong YK, Murugesan A, Vishnupriya K, Ashwin R, Daniel EA, Balakrishnan P, Raju S, Rosmawati M, Velu V, Larsson M, Shankar EM. Vimali J, et al. Front Med (Lausanne). 2022 Nov 3;9:1019230. doi: 10.3389/fmed.2022.1019230. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36405584 Free PMC article.
References
- Martins I, Gomes-Neto F, Faustino A, Carvalho F, Carneiro F, Bozza P, Mohana-Borges R, Castanho M, Almeida F, Santos N, Da Poian A. 2012. The disordered N-terminal region of dengue virus capsid protein contains a lipid droplet-binding motif. Biochem. J. 444:405–415 - PubMed
- Samsa MM, Mondotte JA, Iglesias NG, Assunção-Miranda I, Barbosa-Lima G, Da Poian AT, Bozza PT, Gamarnik AV. 2009. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog. 5:e1000632 doi:10.1371/journal.ppat.1000632 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources