Lysyl oxidase plays a critical role in endothelial cell stimulation to drive tumor angiogenesis - PubMed (original) (raw)
Lysyl oxidase plays a critical role in endothelial cell stimulation to drive tumor angiogenesis
Ann-Marie Baker et al. Cancer Res. 2013.
Abstract
Identification of key molecules that drive angiogenesis is critical for the development of new modalities for the prevention of solid tumor progression. Using multiple models of colorectal cancer, we show that activity of the extracellular matrix-modifying enzyme lysyl oxidase (LOX) is essential for stimulating endothelial cells in vitro and angiogenesis in vivo. We show that LOX activates Akt through platelet-derived growth factor receptor β (PDGFRβ) stimulation, resulting in increased VEGF expression. LOX-driven angiogenesis can be abrogated through targeting LOX directly or using inhibitors of PDGFRβ, Akt, and VEGF signaling. Furthermore, we show that LOX is clinically correlated with VEGF expression and blood vessel formation in 515 colorectal cancer patient samples. Finally, we validate our findings in a breast cancer model, showing the universality of these observations. Taken together, our findings have broad clinical and therapeutic implications for a wide variety of solid tumor types.
Figures
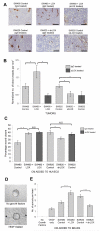
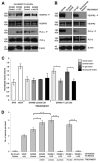
Figure 1. LOX promotes establishment of CD31 positive blood vessels in subcutaneous tumors, and endothelial cell migration and angiogenic sprouting in vitro
(A) Representative immunohistochemical staining of CD31 in sections of SW480 and SW620 tumors grown in nude mice treated with a control IgG or LOX-targeting antibody (‘αLOX’). CD31 is shown in brown, and cell nuclei in blue. Scale bar represents 100μm. (B) Normalized density of CD31 positive blood vessels in SW480 and SW620 subcutaneous tumors grown in nude mice treated with a control IgG or LOX-targeting antibody (‘αLOX’). n ≥ 4 tumors per condition. (C) Quantification of HUVEC migration in the presence of conditioned media collected from SW480 or SW620 CRC cells with manipulated LOX expression. Measurements of wound area were taken at 0 and 8 hours, and used to calculate percentage wound closure. HUVECs were treated with control IgG or LOX targeting antibody (‘αLOX’) at 0 hours. n = 4 per condition. (D) Representative images of the angiogenic sprouting assay. Arrows indicate sprouts of endothelial cells extending from the HUVEC-coated bead. (E) Quantification of in vitro sprouting from HUVEC coated beads treated with negative control (‘No growth factors’), positive control (‘VEGF only’) or conditioned media collected from CRC cell lines. n = 2 wells per condition, 15 beads per well. Bars in (B), (C) and (E) represent mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 using the two sided Student’s t test.
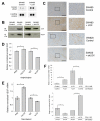
Figure 2. LOX promotes VEGF expression in tumor cells
(A) Secreted VEGF levels in conditioned media collected from SW480 control cells or LOX overexpressing cells (‘SW480 + LOX’) in vitro, detected using a human angiogenesis antibody array. (B) Immunoblot of secreted VEGF and LOX levels in conditioned media collected from SW480 control or LOX overexpressing (‘SW480 + LOX’) cells, and SW620 control or LOX knockdown (‘SW620 + shLOX’) cells in vitro. kDa = kilodalton. (C) Representative immunohistochemical staining of VEGF in sections of SW480 and SW620 subcutaneous tumors grown in nude mice. VEGF is shown in brown, and cell nuclei in blue. Scale bar represents 200μm. (D) Effect of addition of recombinant human LOX (‘huLOX’) or LOX function inhibiting antibody (‘αLOX’) on secretion of VEGF protein in SW480 cells, as determined by ELISA. n = 2 wells per condition. (E) Effect of addition of huLOX or αLOX on VEGF mRNA levels in SW480 cells, as determined by qRT-PCR. n = 3 wells per condition. (F) Effect of extracellular LOX levels on VEGF mRNA in SW480 (upper panel) and SW620 (lower panel) cell lines. Conditioned media collected from cells with manipulated LOX levels were added to cells with manipulated LOX levels as indicated. Cells were incubated for 16 hours before the collection of mRNA and analysis by qRT-PCR. n = 3 wells per condition. Bars in (D), (E) and (F) represent mean ± SEM. *p < 0.05, **p < 0.01 using the two sided Student’s t test.
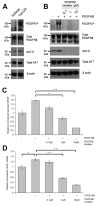
Figure 3. LOX-mediated upregulation of VEGF transcription requires phosphorylation of Akt
(A) Representative immunoblot for phosphorylated Akt(Ser473) (‘Akt-P’) and total Akt in SW480 cell lysates. Conditioned media (‘CM’) with low (‘Control’) or high LOX levels was added to the cells 16 hours prior to analysis. β actin was used as a loading control. kDa = kilodalton. (B) Representative immunoblot for phosphorylated Akt(Ser473) (‘Akt-P’) and total Akt in SW480 cell lysates treated with PBS, recombinant human LOX (‘huLOX’), rabbit IgG control (‘IgG’) or LOX-targeting antibody (‘αLOX’). β actin was used as a loading control. kDa = kilodalton. (C) Representative immunoblot for phosphorylated Akt(Ser473) (‘Akt-P’) and total Akt in SW480 tumor lysates treated with rabbit IgG control (‘IgG’) or LOX-targeting antibody (‘αLOX’) where indicated. β actin was used as a loading control. kDa = kilodalton. (D) Representative immunohistochemical staining of phosphorylated Akt(Ser473) in sections taken from SW480 subcutaneous tumors grown in nude mice. Phosphorylated Akt(Ser473) is shown in brown, and cell nuclei in blue. Scale bar represents 200μm. (E) Representative immunoblot for phosphorylated Akt(Ser473) (‘Akt-P’) and total Akt in SW480 cell lysates treated with the Akt inhibitor MK-2206. β actin was used as a loading control. kDa = kilodalton. (F) Effect of MK-2206 treatment on secretion of VEGF protein in SW480 cells, as determined by ELISA. n = 2 wells per condition. (G) Effect of MK-2206 treatment on VEGF mRNA in SW480 cells, as determined by qRT-PCR. n = 3 wells per condition. Bars in (F) and (G) represent mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 using the two sided Student’s t test.
Figure 4. LOX-dependent PDGFRβ activation upregulates Akt phosphorylation and VEGF secretion
(A) Representative immunoblot for phosphorylated PDGFRβ(Tyr751) (‘PDGFR-P’), total PDGFRβ, phosphorylated Akt(Ser473) (‘Akt-P’) and total Akt in SW480 cell lysates treated with 150ng/ml purified human recombinant LOX (‘huLOX’) for 16 hours prior to lysis. β actin was used as a loading control. kDa = kilodalton. (B) Representative immunoblot for phosphorylated PDGFRβ(Tyr751) (‘PDGFR-P’), total PDGFRβ phosphorylated Akt(Ser473) (‘Akt-P’) and total Akt in serum-starved SW480 LOX-overexpressing cell lysates pretreated with a PDGFRβ inhibitor for 1 hour then stimulated with 25ng/ml PDGF-BB for 2 minutes. β actin was used as a loading control. kDa = kilodalton. (C) Effect of PDGFRβ inhibition on secretion of VEGF protein in SW480 LOX-overexpressing cells. Cells were treated for 16 hours prior to collecting media and analysis by ELISA. n = 2 wells per condition. (D) Effect of PDGFRβ inhibition on VEGF mRNA in SW480 LOX-overexpressing cells. Cells were treated for 16 hours prior to collecting RNA and analysis by qRT-PCR. n = 3 wells per condition. Bars in (C) and (D) represent mean ± SEM. *p < 0.05, **p < 0.01 using the two sided Student’s t test.
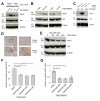
Figure 5. The pro-angiogenic effects of LOX can be prevented in vitro by inhibition of VEGFR2 signaling
(A) Representative immunoblot showing activity of the VEGFR2 signaling pathway in HUVECs upon stimulation with conditioned media (‘CM’) collected from SW480 or SW620 CRC cells. Levels of phosphorylated VEGFR2(Tyr1175) (‘VEGFR2 - P’), VEGFR2, phosphorylated PLC-γ(Tyr783) (‘PLC-γ – P’) and PLC-γ are shown. (B) Representative immunoblot showing activity of the VEGFR2 signaling pathway in HUVECs upon treatment with vehicle control, sunitinib, human IgG control (‘Hu IgG’) or bevacizumab. Levels of phosphorylated VEGFR2(Tyr1175) (‘VEGFR2 - P’), VEGFR2, phosphorylated PLC-γ(Tyr783) (‘PLC-γ – P’) and PLC-γ are shown. β actin was used as a loading control. kDa = kilodalton. (C) Quantification of HUVEC migration in the presence of conditioned media (‘CM’) collected from SW480 CRC cells, and treated with vehicle, sunitinib (100nM), human IgG control (‘IgG’, 50μg/ml) or bevacizumab (50μg/ml) where indicated. Addition of serum free media (‘SFM’) or 50ng/ml human VEGF (‘VEGF’) were used as negative and positive controls respectively. Measurements of wound area were taken at 0 and 8 hours, and used to calculate percentage wound closure in this time. (D) Quantification of in vitro sprouting from HUVEC coated beads treated with negative control (‘No growth factors’), positive control (‘VEGF only’) or conditioned media collected from the SW480 cell line. Vehicle, sunitinib (100nM), human IgG control (‘Hu IgG’, 50μg/ml) or bevacizumab (50μg/ml) were added to the media where indicated. n ≥ 2 wells per condition, 15 beads per well. Bars in (C) and (D) represent mean ± SEM. *p < 0.05, ***p < 0.001 using the two sided Student’s t test.
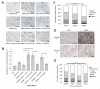
Figure 6. LOX, VEGF and blood vessel formation are correlated in vivo and in patient samples
(A) Representative immunohistochemical staining of endomucin in sections of sponges implanted subcutaneously into mice, and injected three times per week. Negative control injections were serum free medium (SFM) and positive control injections were 10ng/ml murine FGF/VEGF. All other sponges were injected with conditioned media (CM) collected from SW480 CRC cells. Mice were treated systemically with vehicle, sunitinib (40mg/kg), human IgG control (‘Hu IgG’) or bevacizumab (‘beva’) (5mg/kg). Scale bar represents 100μm. (B) Quantification of blood vessel establishment in sponges implanted subcutaneously into mice. Endomucin is shown in brown, and cell nuclei in blue. Bars represent normalized mean number of blood vessels per cm2 ± SEM. n = 4 sponges per condition for negative (SFM) and positive (VEGF/FGF) controls, n = 8 sponges per condition for conditioned media injections. *P < 0.05, **P < 0.01 using the two sided Student’s t test. (C) Distribution of VEGF immunoreactivity in primary CRC tumors, representing stage I (n = 86), stage II (n = 198) and stage III (n = 213), from the patient TMA. Samples were scored as ‘negative’, ‘weak’, ‘moderate’ or ‘strong’, as measured by VEGF immunostaining. ***P < 0.001 by the Pearson Chi Square test. (D) LOX and VEGF immunohistochemical staining in two representative CRC patient tissues (‘Sample 1’ and ‘Sample 2’) taken from the TMA. VEGF and LOX are shown in brown, and cell nuclei in blue. Scale bar represents 50μm. (E) Distribution of LOX and VEGF immunoreactivity in the CRC patient TMA. Samples were scored as ‘negative’, ‘weak’, ‘moderate’ or ‘strong’, as measured by LOX or VEGF immunostaining. **p < 0.01 by the Pearson Chi Square test.
Similar articles
- DDA suppresses angiogenesis and tumor growth of colorectal cancer in vivo through decreasing VEGFR2 signaling.
Huang SW, Lien JC, Kuo SC, Huang TF. Huang SW, et al. Oncotarget. 2016 Sep 27;7(39):63124-63137. doi: 10.18632/oncotarget.11152. Oncotarget. 2016. PMID: 27517319 Free PMC article. - Lysyl oxidase assists tumor‑initiating cells to enhance angiogenesis in hepatocellular carcinoma.
Yang M, Liu J, Wang F, Tian Z, Ma B, Li Z, Wang B, Zhao W. Yang M, et al. Int J Oncol. 2019 Apr;54(4):1398-1408. doi: 10.3892/ijo.2019.4705. Epub 2019 Jan 31. Int J Oncol. 2019. PMID: 30720077 - The HIF-1-inducible lysyl oxidase activates HIF-1 via the Akt pathway in a positive regulation loop and synergizes with HIF-1 in promoting tumor cell growth.
Pez F, Dayan F, Durivault J, Kaniewski B, Aimond G, Le Provost GS, Deux B, Clézardin P, Sommer P, Pouysségur J, Reynaud C. Pez F, et al. Cancer Res. 2011 Mar 1;71(5):1647-57. doi: 10.1158/0008-5472.CAN-10-1516. Epub 2011 Jan 14. Cancer Res. 2011. PMID: 21239473 - Lysyl Oxidase (LOX): Functional Contributions to Signaling Pathways.
Laczko R, Csiszar K. Laczko R, et al. Biomolecules. 2020 Jul 22;10(8):1093. doi: 10.3390/biom10081093. Biomolecules. 2020. PMID: 32708046 Free PMC article. Review. - Lysyl oxidase in colorectal cancer.
Cox TR, Erler JT. Cox TR, et al. Am J Physiol Gastrointest Liver Physiol. 2013 Nov 15;305(10):G659-66. doi: 10.1152/ajpgi.00425.2012. Epub 2013 Sep 5. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 24008360 Review.
Cited by
- LOX expression and functional analysis in astrocytomas and impact of IDH1 mutation.
da Silva R, Uno M, Marie SK, Oba-Shinjo SM. da Silva R, et al. PLoS One. 2015 Mar 19;10(3):e0119781. doi: 10.1371/journal.pone.0119781. eCollection 2015. PLoS One. 2015. PMID: 25790191 Free PMC article. - Matrix stiffening and acquired resistance to chemotherapy: concepts and clinical significance.
Darvishi B, Eisavand MR, Majidzadeh-A K, Farahmand L. Darvishi B, et al. Br J Cancer. 2022 May;126(9):1253-1263. doi: 10.1038/s41416-021-01680-8. Epub 2022 Feb 5. Br J Cancer. 2022. PMID: 35124704 Free PMC article. Review. - Insights into the structure and dynamics of lysyl oxidase propeptide, a flexible protein with numerous partners.
Vallet SD, Miele AE, Uciechowska-Kaczmarzyk U, Liwo A, Duclos B, Samsonov SA, Ricard-Blum S. Vallet SD, et al. Sci Rep. 2018 Aug 6;8(1):11768. doi: 10.1038/s41598-018-30190-6. Sci Rep. 2018. PMID: 30082873 Free PMC article. - MCAM and LAMA4 Are Highly Enriched in Tumor Blood Vessels of Renal Cell Carcinoma and Predict Patient Outcome.
Wragg JW, Finnity JP, Anderson JA, Ferguson HJ, Porfiri E, Bhatt RI, Murray PG, Heath VL, Bicknell R. Wragg JW, et al. Cancer Res. 2016 Apr 15;76(8):2314-26. doi: 10.1158/0008-5472.CAN-15-1364. Epub 2016 Feb 26. Cancer Res. 2016. PMID: 26921326 Free PMC article. - Inhibition of the HIF-1 Survival Pathway as a Strategy to Augment Photodynamic Therapy Efficacy.
de Keijzer MJ, de Klerk DJ, de Haan LR, van Kooten RT, Franchi LP, Dias LM, Kleijn TG, van Doorn DJ, Heger M; Photodynamic Therapy Study Group. de Keijzer MJ, et al. Methods Mol Biol. 2022;2451:285-403. doi: 10.1007/978-1-0716-2099-1_19. Methods Mol Biol. 2022. PMID: 35505024
References
- Subarsky P, Hill RP. The hypoxic tumour microenvironment and metastatic progression. Clin Exp Metastasis. 2003;20:237–50. - PubMed
- Erler JT, Giaccia AJ. Lysyl oxidase mediates hypoxic control of metastasis. Cancer Res. 2006;66:10238–41. - PubMed
- Kagan HM, Trackman PC. Properties and function of lysyl oxidase. Am J Respir Cell Mol Biol. 1991;5:206–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- A10433/CRUK_/Cancer Research UK/United Kingdom
- BREAST CANCER NOW RESEARCH CENTRE/BCN_/Breast Cancer Now/United Kingdom
- C107/A10433/CRUK_/Cancer Research UK/United Kingdom
- G0800102/MRC_/Medical Research Council/United Kingdom
- CSO_/Chief Scientist Office/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical