Targeting PPARγ Signaling Cascade for the Prevention and Treatment of Prostate Cancer - PubMed (original) (raw)
Targeting PPARγ Signaling Cascade for the Prevention and Treatment of Prostate Cancer
Sakshi Sikka et al. PPAR Res. 2012.
Abstract
The peroxisome proliferator-activated receptor-gamma (PPARγ) is a member of the hormone-activated nuclear receptor superfamily. PPARγ can be activated by a diverse group of agents, such as endogenous polyunsaturated fatty acids, 15-deoxy-Δ(12,14)-prostaglandin J(2) (15d-PGJ(2)), and thiazolidinedione (TZD) drugs. PPARγ induces antiproliferative, antiangiogenic, and prodifferentiation pathways in several tissue types, thus making it a highly useful target for downregulation of carcinogenesis. These TZD-derived novel therapeutic agents, alone or in combination with other anticancer drugs, have translational relevance in fostering effective strategies for cancer treatment. TZDs have been proven for antitumor activity in a wide variety of experimental cancer models, both in vitro and in vivo, by affecting the cell cycle, inducing cell differentiation and apoptosis, as well as by inhibiting tumor angiogenesis. Angiogenesis inhibition mechanisms of TZDs include direct inhibition of endothelial cell proliferation and migration, as well as reduction in tumor cell vascular endothelial growth factor production. In prostate cancer, PPARγ ligands such as troglitazone and 15d-PGJ(2) have also shown to inhibit tumor growth. This paper will focus on current discoveries in PPARγ activation, targeting prostate carcinogenesis as well as the role of PPARγ as a possible anticancer therapeutic option. Here, we review PPARγ as an antitumor agent and summarize the antineoplastic effects of PPARγ agonists in prostate cancer.
Figures
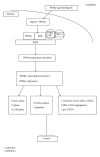
Figure 1
PPAR_γ_ activity. Upon ligand binding PPAR_γ_, binds to RXR in the nucleus and associates with coactivators to bind PPRE located on target genes that control various activities.
Similar articles
- Beyond peroxisome proliferator-activated receptor gamma signaling: the multi-facets of the antitumor effect of thiazolidinediones.
Weng JR, Chen CY, Pinzone JJ, Ringel MD, Chen CS. Weng JR, et al. Endocr Relat Cancer. 2006 Jun;13(2):401-13. doi: 10.1677/erc.1.01182. Endocr Relat Cancer. 2006. PMID: 16728570 Review. - Down-regulation of X-linked inhibitor of apoptosis synergistically enhanced peroxisome proliferator-activated receptor gamma ligand-induced growth inhibition in colon cancer.
Qiao L, Dai Y, Gu Q, Chan KW, Zou B, Ma J, Wang J, Lan HY, Wong BC. Qiao L, et al. Mol Cancer Ther. 2008 Jul;7(7):2203-11. doi: 10.1158/1535-7163.MCT-08-0326. Mol Cancer Ther. 2008. PMID: 18645029 - 15-Deoxy-Delta12,14-prostaglandin J(2) induces death receptor 5 expression through mRNA stabilization independently of PPARgamma and potentiates TRAIL-induced apoptosis.
Nakata S, Yoshida T, Shiraishi T, Horinaka M, Kouhara J, Wakada M, Sakai T. Nakata S, et al. Mol Cancer Ther. 2006 Jul;5(7):1827-35. doi: 10.1158/1535-7163.MCT-06-0023. Mol Cancer Ther. 2006. PMID: 16891469 - Peroxisome proliferator-activated receptor gamma pathway targeting in carcinogenesis: implications for chemoprevention.
Ondrey F. Ondrey F. Clin Cancer Res. 2009 Jan 1;15(1):2-8. doi: 10.1158/1078-0432.CCR-08-0326. Clin Cancer Res. 2009. PMID: 19118026 Review. - Suppression of pancreatic carcinoma growth by activating peroxisome proliferator-activated receptor gamma involves angiogenesis inhibition.
Dong YW, Wang XP, Wu K. Dong YW, et al. World J Gastroenterol. 2009 Jan 28;15(4):441-8. doi: 10.3748/wjg.15.441. World J Gastroenterol. 2009. PMID: 19152448 Free PMC article.
Cited by
- Fatty acid activated PPARγ promotes tumorigenicity of prostate cancer cells by up regulating VEGF via PPAR responsive elements of the promoter.
Forootan FS, Forootan SS, Gou X, Yang J, Liu B, Chen D, Al Fayi MS, Al-Jameel W, Rudland PS, Hussain SA, Ke Y. Forootan FS, et al. Oncotarget. 2016 Feb 23;7(8):9322-39. doi: 10.18632/oncotarget.6975. Oncotarget. 2016. PMID: 26814431 Free PMC article. - Natural compounds targeting nuclear receptors for effective cancer therapy.
Hegde M, Girisa S, Naliyadhara N, Kumar A, Alqahtani MS, Abbas M, Mohan CD, Warrier S, Hui KM, Rangappa KS, Sethi G, Kunnumakkara AB. Hegde M, et al. Cancer Metastasis Rev. 2023 Sep;42(3):765-822. doi: 10.1007/s10555-022-10068-w. Epub 2022 Dec 8. Cancer Metastasis Rev. 2023. PMID: 36482154 Review. - Evodiamine Mitigates Cellular Growth and Promotes Apoptosis by Targeting the c-Met Pathway in Prostate Cancer Cells.
Hwang ST, Um JY, Chinnathambi A, Alharbi SA, Narula AS, Namjoshi OA, Blough BE, Ahn KS. Hwang ST, et al. Molecules. 2020 Mar 13;25(6):1320. doi: 10.3390/molecules25061320. Molecules. 2020. PMID: 32183146 Free PMC article. - Apoptotic Mechanisms of Peroxisome Proliferator-Activated Receptor-γ Activation in Acinar Cells During Acute Pancreatitis.
Xu P, Lou XL, Chen C. Xu P, et al. Pancreas. 2016 Feb;45(2):179-86. doi: 10.1097/MPA.0000000000000495. Pancreas. 2016. PMID: 26495791 Free PMC article. - Systematic, network-based characterization of therapeutic target inhibitors.
Shen Y, Alvarez MJ, Bisikirska B, Lachmann A, Realubit R, Pampou S, Coku J, Karan C, Califano A. Shen Y, et al. PLoS Comput Biol. 2017 Oct 12;13(10):e1005599. doi: 10.1371/journal.pcbi.1005599. eCollection 2017 Oct. PLoS Comput Biol. 2017. PMID: 29023443 Free PMC article.
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer Journal for Clinicians. 2008;58(2):71–96. - PubMed
- Hirsch J, Johnson CL, Nelius T, Kennedy R, Riese WD, Filleur S. PEDF inhibits IL8 production in prostate cancer cells through PEDF receptor/phospholipase A2 and regulation of NFκB and PPARγ . Cytokine. 2011;55(2):202–210. - PubMed
- Lehrke M, Lazar MA. The many faces of PPARγ . Cell. 2005;123(6):993–999. - PubMed
LinkOut - more resources
Full Text Sources