Inhibitory effects of crude extracts from some edible Thai plants against replication of hepatitis B virus and human liver cancer cells - PubMed (original) (raw)
Inhibitory effects of crude extracts from some edible Thai plants against replication of hepatitis B virus and human liver cancer cells
Wanwisa Waiyaput et al. BMC Complement Altern Med. 2012.
Abstract
Background: Edible plants such as Cratoxylum formosum (Jack) Dyer, Curcumin longa Lin, Momordica charantia Lin and Moringa oleifera Lam have long been believed in Thai culture to relieve ulcers and the symptoms of liver disease. However, little is known about their anti-liver cancer properties and antiviral activity against hepatitis B virus (HBV). The aim of this study was to investigate the anti-liver cancer and anti-HBV activities of crude extracts from these edible plants on human liver cancer cells.
Methods: Plant samples were prepared and extracted using buffer and hydro-alcoholic solvents. The MTT assay was performed to investigate the effects of the plant extracts on the cell viability of HepG2 cells. The inhibitory effect on replication of HBV was analysed by determining the level of HBV covalently closed circular DNA (cccDNA) in transiently transfected HepG2 cells with the DNA expression plasmid of the HBV genome using a quantitative real-time PCR.
Results: Buffer and hydroalcoholic extracts from C. formosum (leaf) reduced cell viability of HepG2 cells and they also inhibited HBV cccDNA. Crude extracts from C. longa (bulb) in both solvents did not have any cytotoxic effects on the HepG2 cells, but they significantly decreased the level of HBV cccDNA. Buffer extracts from the leaves of M. charantia and the fruits of M. oleifera showed to have anti-HBV activity and also a mild cytotoxicity effect on the HepG2 cells. In addition, leaves of M. Oleifera extracted by hydroalcoholic solvent drastically decreased the level of cccDNA in transiently transfected HepG2 cells.
Conclusion: Some crude extracts of edible plants contain compounds that demonstrate anti-liver cancer and anti-HBV activities.
Figures
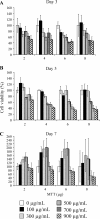
Figure 1
Optimisation experiment of MTT assay on COS-7 cells. Cells were treated with various concentrations of C. longa hot water extract as indicated in quadruplicate. After 3 days (A); 5 days (B); and 7 days (C) of incubation, cells were subjected to viability analysis using MTT assay with different amounts of MTT (2-8 μg). Bar graph represents a mean value whereas an error bar indicates the uncertainty value of three independent experiments.
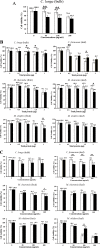
Figure 2
The cytotoxicity effects from plant extracts on COS-7 and HepG2 cells. (A) The effect from hot water extract of C. longa (bulb), (B) The effects from buffer extracts and (C) The effects from 80% hydroalcoholic extracts. The white bars represent the percentage of cell viability of COS-7 cells whereas the black bars represent the percentage of cell viability of HepG2 cells. Bar graph represents a mean value whereas an error bar indicates the uncertainty value of three independent experiments. “*” and “**” indicated significant inhibitory effect when compared with both cell lines at p < 0.01 and p < 0.001 (by t-test) respectively.
Figure 3
Effects of crude extracts on the level of cccDNA in transiently transfected HepG2 cells with the DNA expression plasmid of HBV genome. HepG2 cells were treated with either 30 μg/mL of hydroalcoholic extracts or 0.3 μg of buffer extracts and were transfected with 1 μg of the DNA expression plasmid of HBV genome using Lipofectamine ™ 2000 in triplicate. After 5 days of incubation, the total DNA of each well was extracted and subjected for quantitative real-time analysis. Bar graph represents a mean value whereas an error bar indicates the uncertainty value of three independent experiments. “*” and “**” indicate significant inhibitory effect when compared with the positive cccDNA at p < 0.01 and p < 0.001 (by t- test) respectively. “F” is for fruit and “L” is for leaf.
Similar articles
- Antiviral effect of Curcuma longa Linn extract against hepatitis B virus replication.
Kim HJ, Yoo HS, Kim JC, Park CS, Choi MS, Kim M, Choi H, Min JS, Kim YS, Yoon SW, Ahn JK. Kim HJ, et al. J Ethnopharmacol. 2009 Jul 15;124(2):189-96. doi: 10.1016/j.jep.2009.04.046. Epub 2009 May 3. J Ethnopharmacol. 2009. PMID: 19409970 - Establishment of Cre-mediated HBV recombinant cccDNA (rcccDNA) cell line for cccDNA biology and antiviral screening assays.
Wu M, Li J, Yue L, Bai L, Li Y, Chen J, Zhang X, Yuan Z. Wu M, et al. Antiviral Res. 2018 Apr;152:45-52. doi: 10.1016/j.antiviral.2018.02.007. Epub 2018 Feb 9. Antiviral Res. 2018. PMID: 29432776 - Curcumin inhibits hepatitis B virus infection by down-regulating cccDNA-bound histone acetylation.
Wei ZQ, Zhang YH, Ke CZ, Chen HX, Ren P, He YL, Hu P, Ma DQ, Luo J, Meng ZJ. Wei ZQ, et al. World J Gastroenterol. 2017 Sep 14;23(34):6252-6260. doi: 10.3748/wjg.v23.i34.6252. World J Gastroenterol. 2017. PMID: 28974891 Free PMC article. - [Recent Advances in Hepatitis B Virus Antivirals].
Liu Q, Yao C, Guo X, Hu K. Liu Q, et al. Bing Du Xue Bao. 2016 Sep;32(5):650-8. Bing Du Xue Bao. 2016. PMID: 30004193 Review. Chinese. - Hepatitis B: Model Systems and Therapeutic Approaches.
Yu X, Gao Y, Zhang X, Ji L, Fang M, Li M, Gao Y. Yu X, et al. J Immunol Res. 2024 May 7;2024:4722047. doi: 10.1155/2024/4722047. eCollection 2024. J Immunol Res. 2024. PMID: 38745751 Free PMC article. Review.
Cited by
- Traditional foods with their constituent's antiviral and immune system modulating properties.
Rahman MM, Mosaddik A, Alam AK. Rahman MM, et al. Heliyon. 2021 Jan;7(1):e05957. doi: 10.1016/j.heliyon.2021.e05957. Epub 2021 Jan 14. Heliyon. 2021. PMID: 33462562 Free PMC article. Review. - Soluble extract from Moringa oleifera leaves with a new anticancer activity.
Jung IL. Jung IL. PLoS One. 2014 Apr 18;9(4):e95492. doi: 10.1371/journal.pone.0095492. eCollection 2014. PLoS One. 2014. PMID: 24748376 Free PMC article. - In vitro cytotoxic screening of 31 crude extracts of Thai herbs on a chondrosarcoma cell line and primary chondrocytes and apoptotic effects of selected extracts.
Ruamrungsri N, Siengdee P, Sringarm K, Chomdej S, Ongchai S, Nganvongpanit K. Ruamrungsri N, et al. In Vitro Cell Dev Biol Anim. 2016 Apr;52(4):434-44. doi: 10.1007/s11626-016-0006-4. Epub 2016 Feb 8. In Vitro Cell Dev Biol Anim. 2016. PMID: 26857828 - Antibacterial and Antiproliferative Activities of Plumericin, an Iridoid Isolated from Momordica charantia Vine.
Saengsai J, Kongtunjanphuk S, Yoswatthana N, Kummalue T, Jiratchariyakul W. Saengsai J, et al. Evid Based Complement Alternat Med. 2015;2015:823178. doi: 10.1155/2015/823178. Epub 2015 Apr 7. Evid Based Complement Alternat Med. 2015. PMID: 25945113 Free PMC article. - Antiviral Properties of Moringa oleifera Leaf Extracts against Respiratory Viruses.
Giugliano R, Ferraro V, Chianese A, Della Marca R, Zannella C, Galdiero F, Fasciana TMA, Giammanco A, Salerno A, Cannillo J, Rotondo NP, Lentini G, Cavalluzzi MM, De Filippis A, Galdiero M. Giugliano R, et al. Viruses. 2024 Jul 25;16(8):1199. doi: 10.3390/v16081199. Viruses. 2024. PMID: 39205173 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources