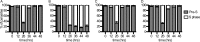Isoprenoid biosynthesis inhibition disrupts Rab5 localization and food vacuolar integrity in Plasmodium falciparum - PubMed (original) (raw)
Isoprenoid biosynthesis inhibition disrupts Rab5 localization and food vacuolar integrity in Plasmodium falciparum
Ruth Howe et al. Eukaryot Cell. 2013 Feb.
Abstract
The antimalarial agent fosmidomycin is a validated inhibitor of the nonmevalonate isoprenoid biosynthesis (methylerythritol 4-phosphate [MEP]) pathway in the malaria parasite, Plasmodium falciparum. Since multiple classes of prenyltransferase inhibitors kill P. falciparum, we hypothesized that protein prenylation was one of the essential functions of this pathway. We found that MEP pathway inhibition with fosmidomycin reduces protein prenylation, confirming that de novo isoprenoid biosynthesis produces the isoprenyl substrates for protein prenylation. One important group of prenylated proteins is small GTPases, such as Rab family members, which mediate cellular vesicular trafficking. We have found that Rab5 proteins dramatically mislocalize upon fosmidomycin treatment, consistent with a loss of protein prenylation. Fosmidomycin treatment caused marked defects in food vacuolar morphology and integrity, consistent with a defect in Rab-mediated vesicular trafficking. These results provide insights to the biological functions of isoprenoids in malaria parasites and may assist the rational selection of secondary agents that will be useful in combination therapy with new isoprenoid biosynthesis inhibitors.
Figures
Fig 1
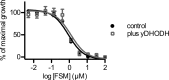
Bypass of electron transport does not confer fosmidomycin resistance. Shown are data for growth inhibition by the isoprenoid biosynthesis inhibitor fosmidomycin (FSM) in P. falciparum parasites (control) compared to parasites that heterologously express yeast dihydroorotate dehydrogenase, which does not require ubiquinone (control + yDHODH). Results are the means and standard deviations from three independent biological replicates.
Fig 2
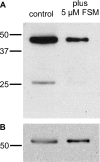
Fosmidomycin treatment inhibits protein prenylation. (A) Antifarnesyl immunoblot of extracts with or without treatment with 5 μM fosmidomycin (FSM) for 24 h. (B) Blot from panel A reprobed with antibodies to Pf-EIF1α to indicate equivalent protein loading. Results are representative of at least three independent biological replicates.
Fig 3
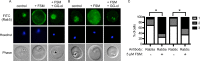
Mislocalization of Rab5 proteins by fosmidomycin treatment. (A and B) Confocal immunofluorescence with either anti-PfRab5a (A) or anti-PfRab5c (B) antibody in untreated parasites (control) compared to fosmidomycin-treated (+FSM) and fosmidomycin- and geranylgeraniol-treated (+FSM +GG-ol) parasites. FITC, fluorescein isothiocyanate. (C) Blinded scoring of >50 cells under each condition for severity of mislocalization of Rab5 (1, typical cellular punctae within parasite; 2, partial mislocalization to erythrocyte or erythrocyte membrane; 3, severe mislocalization to erythrocyte or erythrocyte membrane). ∗, P < 0.001 compared to untreated conditions.
Fig 4
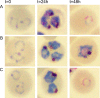
Fosmidomycin treatment causes growth arrest of malaria parasites during schizogony. Shown are Giemsa-stained light micrographs of synchronized ring-stage parasites at the indicated time points of culture for comparison of untreated parasites (A) to those treated with 5 μM fosmidomycin (B) or 5 μM fosmidomycin plus 5 μM the downstream isoprenol geranylgeraniol (C). Images of representative cells are indicative of results from at least five independent biological replicates.
Fig 5
Fosmidomycin-treated parasites arrest during S phase. Shown are proportions of cells with unreplicated DNA (pre-S phase, morphologically early-ring-stage parasites, 12 h after invasion) compared to those in which DNA replication has begun (S phase), as determined by acridine orange staining and flow cytometric evaluation. Untreated parasites (A) are compared to fosmidomycin-treated parasites (B), fosmidomycin- and geranylgeraniol-treated parasites (C), and parasites with geranylgeraniol treatment alone (D). The cell cycle duration under these conditions is approximately 48 h; untreated parasites return to pre-S phase at 36 h.
Fig 6
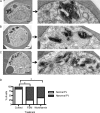
Food vacuolar defect in fosmidomycin- and wortmannin-treated parasites. (A to C) Transmission electron microscopic evaluation of control parasites (A) compared to parasites treated for 24 h with either the isoprenoid inhibitor fosmidomycin (B) or the PI3-K inhibitor wortmannin (C). On the right is a magnified view of a hemozoin-containing FV. (D) Scoring of electron micrographs of control versus fosmidomycin (FSM)- and wortmannin-treated cells. Abnormal FVs were defined as FVs that either lacked an FV membrane or contained more than one discontiguous membrane-bound collection of hemozoin (n > 25 under each condition). ∗, P < 0.001 compared to untreated conditions (Fisher's 2-tailed test).
Fig 7
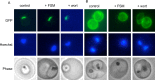
Loss of food vacuolar integrity upon fosmidomycin and wortmannin treatment. Shown is the confocal fluorescence microscopic localization of a plasmepsin II-GFP (P2-GFP) construct (left) or live fluorescence imaging of Lysotracker Red-stained malaria parasites (right). Control parasites (A) are compared to parasites treated for 24 h with either fosmidomycin (B), fosmidomycin plus geranylgeraniol (C), or wortmannin (D). Images are representative of at least three independent biological experiments. Visualization of PMII-GFP in FSM- and wortmannin-treated parasites required higher detector gain levels, resulting in increased observed erythrocyte autofluorescence.
Fig 8
Apicoplast and parasitophorous vacuolar targeting in fosmidomycin- and wortmannin-treated parasites. Shown is live-cell fluorescence of malaria parasites that express either the leader sequence (ACPL-GFP) (A) or signal sequence (ACPs-GFP) (B) from P. falciparum acyl carrier protein, fused to GFP, which traffic to the apicoplast or parasitophorous vacuole, respectively (27). Untreated parasites (control) are compared to fosmidomycin (+FSM)- and wortmannin (+wort)-treated parasites. Images are representative of at least three independent biological experiments.
Fig 9
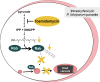
Model of fosmidomycin effects on malaria parasites. Fosmidomycin blocks isoprenoid biosynthesis and causes a defect in growth and vesicular trafficking to the food vacuole (FV). These effects are rescued by geranylgeraniol (GG-ol), indicating that the essential isoprenoids in malaria are metabolically derived from geranylgeranyl pyrophosphate (GG-PP). GG-PP is the substrate for geranylgeranyltransferase (GGTase), which modifies the endocytosis regulator Rab5 (a small GTPase) in most eukaryotes. Blocking of isoprenoid biosynthesis (with fosmidomycin) decreases protein prenylation, causes Rab5 mislocalization, and alters FV morphology. IPP, isopentenyl pyrophosphate; DMAPP, dimethylallyl pyrophosphate.
Similar articles
- The methylerythritol phosphate pathway is functionally active in all intraerythrocytic stages of Plasmodium falciparum.
Cassera MB, Gozzo FC, D'Alexandri FL, Merino EF, del Portillo HA, Peres VJ, Almeida IC, Eberlin MN, Wunderlich G, Wiesner J, Jomaa H, Kimura EA, Katzin AM. Cassera MB, et al. J Biol Chem. 2004 Dec 10;279(50):51749-59. doi: 10.1074/jbc.M408360200. Epub 2004 Sep 27. J Biol Chem. 2004. PMID: 15452112 - Isoprenoid biosynthesis in Plasmodium falciparum.
Guggisberg AM, Amthor RE, Odom AR. Guggisberg AM, et al. Eukaryot Cell. 2014 Nov;13(11):1348-59. doi: 10.1128/EC.00160-14. Epub 2014 Sep 12. Eukaryot Cell. 2014. PMID: 25217461 Free PMC article. Review. - Effect of fosmidomycin on metabolic and transcript profiles of the methylerythritol phosphate pathway in Plasmodium falciparum.
Cassera MB, Merino EF, Peres VJ, Kimura EA, Wunderlich G, Katzin AM. Cassera MB, et al. Mem Inst Oswaldo Cruz. 2007 Jun;102(3):377-83. doi: 10.1590/s0074-02762007000300019. Mem Inst Oswaldo Cruz. 2007. PMID: 17568945 - A sugar phosphatase regulates the methylerythritol phosphate (MEP) pathway in malaria parasites.
Guggisberg AM, Park J, Edwards RL, Kelly ML, Hodge DM, Tolia NH, Odom AR. Guggisberg AM, et al. Nat Commun. 2014 Jul 24;5:4467. doi: 10.1038/ncomms5467. Nat Commun. 2014. PMID: 25058848 Free PMC article. - Apicoplast Metabolism: Parasite's Achilles' Heel.
Kadian K, Gupta Y, Singh HV, Kempaiah P, Rawat M. Kadian K, et al. Curr Top Med Chem. 2018;18(22):1987-1997. doi: 10.2174/1568026619666181130134742. Curr Top Med Chem. 2018. PMID: 30499407 Review.
Cited by
- In vivo antimalarial activity and mechanisms of action of 4-nerolidylcatechol derivatives.
Rocha e Silva LF, Nogueira KL, Pinto AC, Katzin AM, Sussmann RA, Muniz MP, de Andrade Neto VF, Chaves FC, Coutinho JP, Lima ES, Krettli AU, Tadei WP, Pohlit AM. Rocha e Silva LF, et al. Antimicrob Agents Chemother. 2015;59(6):3271-80. doi: 10.1128/AAC.05012-14. Epub 2015 Mar 23. Antimicrob Agents Chemother. 2015. PMID: 25801563 Free PMC article. - Characterization of Domiphen Bromide as a New Fast-Acting Antiplasmodial Agent Inhibiting the Apicoplastidic Methyl Erythritol Phosphate Pathway.
Biosca A, Ramírez M, Gomez-Gomez A, Lafuente A, Iglesias V, Pozo OJ, Imperial S, Fernàndez-Busquets X. Biosca A, et al. Pharmaceutics. 2022 Jun 22;14(7):1320. doi: 10.3390/pharmaceutics14071320. Pharmaceutics. 2022. PMID: 35890216 Free PMC article. - The Plasmodium falciparum ABC transporter ABCI3 confers parasite strain-dependent pleiotropic antimalarial drug resistance.
Murithi JM, Deni I, Pasaje CFA, Okombo J, Bridgford JL, Gnädig NF, Edwards RL, Yeo T, Mok S, Burkhard AY, Coburn-Flynn O, Istvan ES, Sakata-Kato T, Gomez-Lorenzo MG, Cowell AN, Wicht KJ, Le Manach C, Kalantarov GF, Dey S, Duffey M, Laleu B, Lukens AK, Ottilie S, Vanaerschot M, Trakht IN, Gamo FJ, Wirth DF, Goldberg DE, Odom John AR, Chibale K, Winzeler EA, Niles JC, Fidock DA. Murithi JM, et al. Cell Chem Biol. 2022 May 19;29(5):824-839.e6. doi: 10.1016/j.chembiol.2021.06.006. Epub 2021 Jul 6. Cell Chem Biol. 2022. PMID: 34233174 Free PMC article. - Metabolic Survival Adaptations of Plasmodium falciparum Exposed to Sublethal Doses of Fosmidomycin.
Tewari SG, Rajaram K, Swift RP, Reifman J, Prigge ST, Wallqvist A. Tewari SG, et al. Antimicrob Agents Chemother. 2021 Mar 18;65(4):e02392-20. doi: 10.1128/AAC.02392-20. Print 2021 Mar 18. Antimicrob Agents Chemother. 2021. PMID: 33495219 Free PMC article. - Inhibitory Effects of Fosmidomycin Against Babesia microti in vitro.
Wang S, Li M, Luo X, Yu L, Nie Z, Liu Q, An X, Ao Y, Liu Q, Chen J, Tian Y, Zhao J, He L. Wang S, et al. Front Cell Dev Biol. 2020 Apr 28;8:247. doi: 10.3389/fcell.2020.00247. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32411701 Free PMC article.
References
- Sachs J, Malaney P. 2002. The economic and social burden of malaria. Nature 415:680–685 - PubMed
- Aregawi M, Cibulskis R, Kita Y, Otten H, Williams R, World Health Organization. 2010. World malaria report 2010. World Health Organization, Geneva, Switzerland
- Baird JK. 2005. Effectiveness of antimalarial drugs. N. Engl. J. Med. 352:1565–1577 - PubMed
- Olliaro P. 2005. Drug resistance hampers our capacity to roll back malaria. Clin. Infect. Dis. 41(Suppl 4):S247–S257 doi:10.1086/430785 - DOI - PubMed
- Dondorp A, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:455–467 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources