Nivalenol and deoxynivalenol affect rat intestinal epithelial cells: a concentration related study - PubMed (original) (raw)
Nivalenol and deoxynivalenol affect rat intestinal epithelial cells: a concentration related study
Giuseppe Bianco et al. PLoS One. 2012.
Abstract
The integrity of the gastrointestinal tract represents a crucial first level defence against ingested toxins. Among them, Nivalenol is a trichotecenes mycotoxin frequently found on cereals and processed grains; when it contaminates human food and animal feed it is often associated with another widespread contaminant, Deoxynivalenol. Following their ingestion, intestinal epithelial cells are exposed to concentrations of these trichothecenes high enough to cause mycotoxicosis. In this study we have investigated the effects of Nivalenol and Deoxynivalenol on intestinal cells in an in vitro model system utilizing the non-tumorigenic rat intestinal epithelial cell line IEC-6. Both Nivalenol and Deoxynivalenol (5-80 µM) significantly affected IEC-6 viability through a pro-apoptotic process which mainly involved the following steps: (i) Bax induction; (ii) Bcl-2 inhibition, and (iii) caspase-3 activation. Moreover, treatment with Nivalenol produced a significant cell cycle arrest of IEC-6 cells, primarily at the G(0)/G(1) interphase and in the S phase, with a concomitant reduction in the fraction of cells in G(2). Interestingly, when administered at lower concentrations (0.1-2.5 µM), both Nivalenol and Deoxynivalenol affected epithelial cell migration (restitution), representing the initial step in gastrointestinal wound healing in the gut. This reduced motility was associated with significant remodelling of the actin cytoskeleton, and changes in expression of connexin-43 and focal adhesion kinase. The concentration range of Nivalenol or Deoxynivalenol we have tested is comparable with the mean estimated daily intake of consumers eating contaminated food. Thus, our results further highlight the risks associated with intake of even low levels of these toxins.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
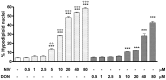
Figure 1. Apoptosis detection by propidium iodide (PI) staining of hypodiploid nuclei after IEC-6 incubation with NIV or DON (0.5–80 µM) for 24 h.
Both NIV and DON exibited a significant and concentration-related pro-apoptotic effect on IEC-6 at concentrations higher than 5 µM and 10 µM respectively (***P<0.001 vs control). NIV at the concentrations between 5 and 80 µM exibits a stronger pro-apoptotic effect compared to the same concentrations of DON (°°°P<0.001, °°P<0.01 vs DON). Data are expressed mean ± s.e.m. from at least three-independent experiments.
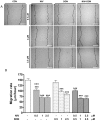
Figure 2. Apoptotic nuclear morphological changes highlighted by DAPI staining in cells treated with graded concentration of NIV and DON (0.5–20 µM) for 24 h.
Apoptotic cells showed pyknotic nuclei and apoptotic bodies were clearly identified after incubation with higher concentration of NIV while DON induces a less marked effect. Cultures were examined and photographed using a confocal microscope as described in method section.
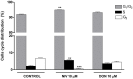
Figure 3. Flow cytometric analysis of IEC-6 cycle phase distribution.
Cells were treated with either NIV or DON (10 µM) for 24 h, incubated with PI and analysed for cell cycle analysis using a Becton Dickinson FACScan flow cytometer and ModFit software (***P<0.001,**P<0.01 vs control). Data are expressed mean ± s.e.m. from at least three-independent experiments.
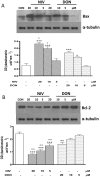
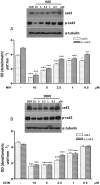
Figure 4. Concentration dependent effect in NIV and DON treated cells on apoptosis-related proteins Bax (panel A) and Bcl-2 (panel B) expression.
Cells were treated with increasing concentration of NIV or DON (5–20 µM) for 24 h and protein expression was detected by Western blot. Tubulin protein expression was used as a loading control. (***P<0.001,**P<0.01,*P<0.05 vs control; °°P<0.01,°P<0.05 vs DON). Western blot is representative of at least three-independent experiments.
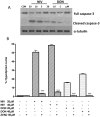
Figure 5. Concentration dependent effect in NIV and DON treated cells on caspase-3 (full and cleaved form) expression.
Cells were treated with increasing concentration of NIV or DON (5–20 µM) for 24 h and protein expression was detected by Western blot. Tubulin protein expression was used as a loading control. The Western blot is representative of independent experiments (panel A). Apoptosis detection by propidium iodide (PI) staining of hypodiploid nuclei with a broad spectrum caspase inhibitor (zVAD-fmk) (panel B). IEC-6 were treated with NIV or DON (20 and 40 µM) for 24 h in presence of zVAD-fmk (50 µM). The pro-apoptotic effect of NIV or DON was significantly reduced by the caspase inhibitor (***P<0.001 NIV+zVAD vs NIV alone; ***P<0.001 DON+zVAD vs DON alone). Data are expressed mean ± s.e.m. from at least three-independent experiments.
Figure 6. Representative pictures of wound repair from mechanical scratch 24 h after NIV and DON cells treatment (0.5–2.5 µM).
Black dotted-line indicates the edge of the wounded area at the starting time (panel A). Quantitative analysis of the wound repair 24 h after making the scratch. For each condition ten different cells were randomly selected to measure the migration distances covered every 10 min from the initial time up to the end of incubation time (***P<0.001 vs control; °°°P<0.001 vs DON; panel B). Data are expressed mean ± s.e.m. from at least three-independent experiments. Bar = 200 µM.
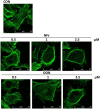
Figure 7. Effect of mycotoxins NIV and DON on IEC-6 cytoskeleton highlighted by FITC-coniugated phalloidin staining.
The microtubule cytoskeleton is displayed in control IEC-6. The microtubules are evident as actin filaments that originate near the nucleus and radiate through the cytosol. Incubation for 24 h of cultured cells with NIV and DON (0.5–2.5 µM) caused a reorganisation of actin filaments characterised by redistribution to the cell subcortical compartment and subsequent cell rounding in concentration dependent manner. Cultures were examined and photographed using a confocal microscope as described in method section.
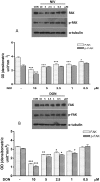
Figure 8. Concentration dependent effect in NIV (Panel A) and DON (Panel B) treated cells on Cx43and its phosphorilated form (pCx43) expression.
Cells were treated with increasing concentration of NIV or DON (0.5–10 µM) for 24 h and protein expression was detected by Western blot (***P<0.001,*P<0.05 vs control; °°°P<0.001,°°P<0.05 vs DON). Tubulin protein expression was used as a loading control. The Western blot is representative of at least three-independent experiments.
Figure 9. Concentration dependent effect in NIV (Panel A) and DON (Panel B) treated cells on FAK and its phosphorilated form expression (pFAK).
Cells were treated with increasing concentration of NIV or DON (0.5–10 µM) for 24 h and protein expression was detected by Western blot. (***P<0.001, **P<0.01, *P<0.05 vs control; °°°P<0.001, °P<0.05 vs DON). Tubulin protein expression was used as a loading control. The Western blot is representative of at least three-independent experiments.
Similar articles
- Nivalenol induces oxidative stress and increases deoxynivalenol pro-oxidant effect in intestinal epithelial cells.
Del Regno M, Adesso S, Popolo A, Quaroni A, Autore G, Severino L, Marzocco S. Del Regno M, et al. Toxicol Appl Pharmacol. 2015 Jun 1;285(2):118-27. doi: 10.1016/j.taap.2015.04.002. Epub 2015 Apr 14. Toxicol Appl Pharmacol. 2015. PMID: 25882925 - Vulnerability of polarised intestinal porcine epithelial cells to mycotoxin deoxynivalenol depends on the route of application.
Diesing AK, Nossol C, Dänicke S, Walk N, Post A, Kahlert S, Rothkötter HJ, Kluess J. Diesing AK, et al. PLoS One. 2011 Feb 25;6(2):e17472. doi: 10.1371/journal.pone.0017472. PLoS One. 2011. PMID: 21364771 Free PMC article. - New insights into mycotoxin mixtures: the toxicity of low doses of Type B trichothecenes on intestinal epithelial cells is synergistic.
Alassane-Kpembi I, Kolf-Clauw M, Gauthier T, Abrami R, Abiola FA, Oswald IP, Puel O. Alassane-Kpembi I, et al. Toxicol Appl Pharmacol. 2013 Oct 1;272(1):191-8. doi: 10.1016/j.taap.2013.05.023. Epub 2013 Jun 2. Toxicol Appl Pharmacol. 2013. PMID: 23735874 - Impacts of the feed contaminant deoxynivalenol on the intestine of monogastric animals: poultry and swine.
Ghareeb K, Awad WA, Böhm J, Zebeli Q. Ghareeb K, et al. J Appl Toxicol. 2015 Apr;35(4):327-37. doi: 10.1002/jat.3083. Epub 2014 Oct 28. J Appl Toxicol. 2015. PMID: 25352520 Review. - Effect of deoxynivalenol and other Type B trichothecenes on the intestine: a review.
Pinton P, Oswald IP. Pinton P, et al. Toxins (Basel). 2014 May 21;6(5):1615-43. doi: 10.3390/toxins6051615. Toxins (Basel). 2014. PMID: 24859243 Free PMC article. Review.
Cited by
- Syzygium cumini (L.) Extract-Derived Green Titanium Dioxide Nanoparticles Induce Caspase-Dependent Apoptosis in Hepatic Cancer Cells.
Rayzah M, Elderdery AY, Alzerwi NAN, Alzahrani B, Alsrhani A, Alsultan A, Idrees B, Rayzah F, Bakhsh Y, Alzahrani AM, Subbiah SK, Mok PL. Rayzah M, et al. Plants (Basel). 2023 Sep 5;12(18):3174. doi: 10.3390/plants12183174. Plants (Basel). 2023. PMID: 37765338 Free PMC article. - Premature ovarian insufficiency: a review on the role of oxidative stress and the application of antioxidants.
Shi YQ, Zhu XT, Zhang SN, Ma YF, Han YH, Jiang Y, Zhang YH. Shi YQ, et al. Front Endocrinol (Lausanne). 2023 Aug 1;14:1172481. doi: 10.3389/fendo.2023.1172481. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37600717 Free PMC article. Review. - Anti-inflammatory Activity of Tanshinone-Related Diterpenes from Perovskia artemisioides Roots.
Sadeghi Z, Cerulli A, Marzocco S, Moridi Farimani M, Masullo M, Piacente S. Sadeghi Z, et al. J Nat Prod. 2023 Apr 28;86(4):812-821. doi: 10.1021/acs.jnatprod.2c01004. Epub 2023 Apr 11. J Nat Prod. 2023. PMID: 37040078 Free PMC article. - Investigation of metabolic crosstalk between host and pathogenic Clostridioides difficile via multiomics approaches.
Kwon JE, Jo SH, Song WS, Lee JS, Jeon HJ, Park JH, Kim YR, Baek JH, Kim MG, Kwon SY, Kim JS, Yang YH, Kim YG. Kwon JE, et al. Front Bioeng Biotechnol. 2022 Sep 2;10:971739. doi: 10.3389/fbioe.2022.971739. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36118584 Free PMC article. - Nivalenol Mycotoxin Concerns in Foods: An Overview on Occurrence, Impact on Human and Animal Health and Its Detection and Management Strategies.
Kumar P, Mahato DK, Gupta A, Pandey S, Paul V, Saurabh V, Pandey AK, Selvakumar R, Barua S, Kapri M, Kumar M, Kaur C, Tripathi AD, Gamlath S, Kamle M, Varzakas T, Agriopoulou S. Kumar P, et al. Toxins (Basel). 2022 Jul 31;14(8):527. doi: 10.3390/toxins14080527. Toxins (Basel). 2022. PMID: 36006189 Free PMC article. Review.
References
- Albassam M, Yong S, Bhatnagar R, Sharma A, Prior M (1987) Histopathologic and electron microscopic studies on the acute toxicity of ochratoxin A in rats. Vet Pathol 24, 427–435. - PubMed
- Azcona-Olivera J, Ouang J, Murtha J, Chu F, Pestka J (1995) Induction of cytokine mRNAs in mice after oral exposure to the trichothecene vomitoxin (deoxynivalenol): relationship to toxin distribuition and protein synthesis inhibition. Toxicol Appl Pharmacol 133, 109–120. - PubMed
- Heussener AH, Dietrich DR, O’Brien E (2006) In vitro investigation of individual and combined cytotoxic effects of ochratoxin A and other selected mycotoxins on renal cells. Toxicol In Vitro 20, 332–41. - PubMed
- Ueno Y (1985) The toxicology of mycotoxins. Crit Rev Toxicol 14, 99–132. - PubMed
- Schorthorst RC, Van Egmond HP (2004) Report from SCOOP task 3.2.10 “collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU member states”. Subtask: trichothecenes. Toxicol Lett 153, 133–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials