Characterization of human brown adipose tissue by chemical-shift water-fat MRI - PubMed (original) (raw)
Characterization of human brown adipose tissue by chemical-shift water-fat MRI
Houchun H Hu et al. AJR Am J Roentgenol. 2013 Jan.
Abstract
Objective: The purpose of this study was to characterize human brown adipose tissue (BAT) with chemical-shift water-fat MRI and to determine whether trends and differences in fat-signal fractions and T2(*) relaxation times between BAT and white adipose tissue (WAT) are consistently observed postmortem and in vivo in infants, adolescents, and adults.
Materials and methods: A postmortem body and eight patients were studied. A six-echo spoiled gradient-echo chemical-shift water-fat MRI sequence was performed at 3 T to jointly quantify fat-signal fraction and T2(*) in interscapular-supraclavicular BAT and subcutaneous WAT. To confirm BAT identity, biopsy and histology served as the reference in the postmortem study and PET/CT was used in five of the eight patients who required examination for medical care.
Results: Fat-signal fractions and T2(*) times were lower in BAT than in WAT in the postmortem example and in seven of eight patients. With the exception of one case, nominal comparisons between brown and white adipose tissues were statistically significant (p < 0.05). Between subjects, a large range of fat-signal fraction values was observed in BAT but not in WAT.
Conclusion: We have shown that fat-signal fractions and T2(*) values jointly derived from chemical-shift water-fat MRI are lower in BAT than in WAT likely because of differences in cellular structures, triglyceride content, and vascularization. The two metrics can serve as complementary biomarkers in the detection of BAT.
Figures
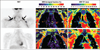
Fig. 1
Coronal MRI results from 3-year-old boy postmortem. Gray-scale water image (left), fat-signal fraction map (middle), and T2* map (right) illustrate bilateral brown adipose tissue (BAT) depots (outlines) and subcutaneous white adipose tissue (WAT) (arrowheads). Fat-signal fraction is illustrated using full 0–100% scale. The T2* map is illustrated using scale condensed from 0 to 26 ms for clarity; any tissue with computed T2* value ≥ 26 ms is shown in red. Compared with subcutaneous WAT, note that BAT is characterized by brighter signal and more heterogeneous and granular appearance on water image, lower fat-signal fractions, and slightly shorter T2* values.
Fig. 2
Photomicrograph from histologic specimen (H and E, ×10) in 3-year-old boy postmortem (same as in Fig. 1). White arrowheads point to large white adipocytes, each characterized by single intracellular vacuole of triglycerides. In contrast, black arrows point to brown adipocytes, which are smaller and exhibit multiple intracellular triglyceride vacuoles. Dotted box shows area where BAT and WAT adipocytes are intermixed.
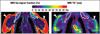
Fig. 3
Imaging results from adolescent patients 2 and 3. In patient 2, 18-year-old woman who was brown adipose tissue–positive (BAT+) (top row), PET data (left) highlight evident uptake of radionuclide tracer by BAT (arrows). Fat-signal fraction (middle) and T2* (right) maps from chemical-shift water-fat MRI are shown. BAT (outlines) is characterized by lower fat-signal fraction and T2* values in contrast with lipid-rich subcutaneous white adipose tissue (WAT) (arrowheads). In patient 3, 18-year-old man who was BAT− (bottom row), PET data show no evident uptake of radionuclide tracer by BAT. However, fat-signal fraction and T2* maps show consistently lower values for BAT (outlines) than subcutaneous WAT (arrowheads).
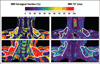
Fig. 4
Imaging results from adult patient 4, 26-year-old man who was brown adipose tissue–positive (BAT+), and patient 5, 49-year-old woman who was BAT−. PET results are not shown. Fat-signal fraction (left) and T2* (right) color maps are shown using same nominal 0–100 scale to highlight differences. Scale selection is arbitrary and was chosen for visual clarity. Supraclavicular and interscapular fat pads exhibit lower fat-signal fractions and T2* values in patient 4 (lean) versus patient 5 (moderately overweight). Particularly in patient 5, imaging appearance of BAT depots appears similar to neighboring subcutaneous white adipose tissue.
Fig. 5
Representative imaging results from pediatric patients. In patient 6, 4-month-old boy, lower fat-signal fraction (left) and T2* (right) values of brown adipose tissue (outlines) are evident in comparison with subcutaneous white adipose tissue (arrowheads). Similar results were obtained from patients 7 and 8 who were 3 months and 5 years old, respectively.
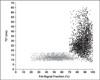
Fig. 6
Voxel scatterplot graph shows fat-signal fraction and T2* region-of-interest measurements from brown adipose tissue (BAT) (gray) and white adipose tissue (WAT) (black) for patient 2. Crosses pinpoint means and reflect SD of each distribution along both nominal dimensions. It is evident that BAT occupies distribution with lower fat-signal fraction and T2* values than WAT.
Similar articles
- Quantification of brown and white adipose tissue based on Gaussian mixture model using water-fat and T2* MRI in adolescents.
Hui SCN, Ko JKL, Zhang T, Shi L, Yeung DKW, Wang D, Chan Q, Chu WCW. Hui SCN, et al. J Magn Reson Imaging. 2017 Sep;46(3):758-768. doi: 10.1002/jmri.25632. Epub 2017 Jan 16. J Magn Reson Imaging. 2017. PMID: 28092409 - Discrimination Between Brown and White Adipose Tissue Using a 2-Point Dixon Water-Fat Separation Method in Simultaneous PET/MRI.
Franz D, Karampinos DC, Rummeny EJ, Souvatzoglou M, Beer AJ, Nekolla SG, Schwaiger M, Eiber M. Franz D, et al. J Nucl Med. 2015 Nov;56(11):1742-7. doi: 10.2967/jnumed.115.160770. Epub 2015 Aug 13. J Nucl Med. 2015. PMID: 26272809 - Comparison of brown and white adipose tissues in infants and children with chemical-shift-encoded water-fat MRI.
Hu HH, Yin L, Aggabao PC, Perkins TG, Chia JM, Gilsanz V. Hu HH, et al. J Magn Reson Imaging. 2013 Oct;38(4):885-96. doi: 10.1002/jmri.24053. Epub 2013 Feb 25. J Magn Reson Imaging. 2013. PMID: 23440739 Free PMC article. - Molecular Imaging of Brown Adipose Tissue Mass.
Yang J, Zhang H, Parhat K, Xu H, Li M, Wang X, Ran C. Yang J, et al. Int J Mol Sci. 2021 Aug 30;22(17):9436. doi: 10.3390/ijms22179436. Int J Mol Sci. 2021. PMID: 34502347 Free PMC article. Review. - Do estrogens enhance activation of brown and beiging of adipose tissues?
Frank AP, Palmer BF, Clegg DJ. Frank AP, et al. Physiol Behav. 2018 Apr 1;187:24-31. doi: 10.1016/j.physbeh.2017.09.026. Epub 2017 Oct 6. Physiol Behav. 2018. PMID: 28988965 Review.
Cited by
- Rotator cuff tear degeneration and the role of fibro-adipogenic progenitors.
Agha O, Diaz A, Davies M, Kim HT, Liu X, Feeley BT. Agha O, et al. Ann N Y Acad Sci. 2021 Apr;1490(1):13-28. doi: 10.1111/nyas.14437. Epub 2020 Jul 29. Ann N Y Acad Sci. 2021. PMID: 32725671 Free PMC article. Review. - Brown fat activity determined by infrared thermography and thermogenesis measurement using whole body calorimetry (BRIGHT Study).
Tay SH, Goh HJ, Govindharajulu P, Cheng J, Camps SG, Haldar S, Velan SS, Sun L, Li Y, Henry CJ, Leow MK. Tay SH, et al. Physiol Res. 2020 Feb 19;69(1):85-97. doi: 10.33549/physiolres.934190. Epub 2019 Dec 19. Physiol Res. 2020. PMID: 31852199 Free PMC article. - MRI assessment of changes in adipose tissue parameters after bariatric surgery.
Lehmann S, Linder N, Retschlag U, Schaudinn A, Stange R, Garnov N, Dietrich A, Oberbach A, Kahn T, Busse H. Lehmann S, et al. PLoS One. 2018 Nov 2;13(11):e0206735. doi: 10.1371/journal.pone.0206735. eCollection 2018. PLoS One. 2018. PMID: 30388152 Free PMC article. - Magnetic Resonance Imaging for Drug Development.
Kim JK. Kim JK. Adv Exp Med Biol. 2021;1310:187-209. doi: 10.1007/978-981-33-6064-8_9. Adv Exp Med Biol. 2021. PMID: 33834438 - Fluorescence Imaging of Interscapular Brown Adipose Tissue in Living Mice.
Rice DR, White AG, Leevy WM, Smith BD. Rice DR, et al. J Mater Chem B. 2015 Mar 7;3(9):1979-1989. doi: 10.1039/C4TB01914H. J Mater Chem B. 2015. PMID: 26015867 Free PMC article.
References
- Cinti S. The role of brown adipose tissue in human obesity. Nutr Metab Cardiovasc Dis. 2006;16:569–574. - PubMed
- Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. - PubMed
- Enerbäck S. Human brown adipose tissue. Cell Metab. 2010;11:248–252. - PubMed
- Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab. 2010;11:268–272. - PubMed
- Baba S, Jacene HA, Engles JM, Honda H, Wahl RL. CT Hounsfield units of brown adipose tissue increase with activation: preclinical and clinical studies. J Nucl Med. 2010;51:246–250. - PubMed
Publication types
MeSH terms
Grants and funding
- K25 DK087931/DK/NIDDK NIH HHS/United States
- R21 DK090778/DK/NIDDK NIH HHS/United States
- K25DK087931/DK/NIDDK NIH HHS/United States
- R21DK090778/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical