Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA - PubMed (original) (raw)
Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA
Jiaxi Wu et al. Science. 2013.
Abstract
Cytosolic DNA induces type I interferons and other cytokines that are important for antimicrobial defense but can also result in autoimmunity. This DNA signaling pathway requires the adaptor protein STING and the transcription factor IRF3, but the mechanism of DNA sensing is unclear. We found that mammalian cytosolic extracts synthesized cyclic guanosine monophosphate-adenosine monophosphate (cyclic GMP-AMP, or cGAMP) in vitro from adenosine triphosphate and guanosine triphosphate in the presence of DNA but not RNA. DNA transfection or DNA virus infection of mammalian cells also triggered cGAMP production. cGAMP bound to STING, leading to the activation of IRF3 and induction of interferon-β. Thus, cGAMP functions as an endogenous second messenger in metazoans and triggers interferon production in response to cytosolic DNA.
Figures
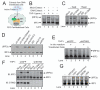
Figure 1. DNA-dependent generation of a heat-resistant small molecule activates the STING pathway
(A) Illustration of an activity assay for cellular factors that activate the STING pathway. PFO: perfringolysin O. (B) Cytosolic extracts from mock or ISD-transfected L929-shSTING cells were incubated with PFO permeabilized THP1 cells together with 35S-IRF3. Dimerization of IRF3 was analyzed by native gel electrophoresis followed by autoradiography. (C) Similar to (B), except that in lanes 4-6, cytosolic extracts were heated at 95°C for 5 min to denature proteins and then the heat-resistant supernatant was incubated with PFO-permeabilized THP1 cells. HT-DNA: herring testis DNA. (D) L929-shSTING cytosolic extracts were incubated with the indicated nucleic acids in the presence of ATP and then the heat-resistant supernatant was assayed for its ability to stimulate IRF3 dimerization in permeabilized Raw264.7 cells. (E) THP1 cells stably expressing shRNA against GFP (control) or STING were permeabilzed with PFO and then incubated with the heat-resistant supernatant from the reaction mixture containing DNA-supplemented L929 cytosolic extracts (lanes 2 and 5) or from DNA-transfected L929 cells (lanes 3 and 6). IRF3 activation was analyzed by native gel electrophoresis. (F) THP1 cells described in (E) were transfected with HT-DNA, poly[I:C] or infected with Sendai virus (SeV), followed by measurement of IRF3 dimerization. (G) Cytosolic extracts from the indicated cell lines were incubated with HT-DNA and then heat-resistant supernatants were assayed for their ability to stimulate IRF3 dimerization in permeabilized Raw264.7 cells. Unless noted otherwise, all results in this paper were representative of at least two independent experiments.
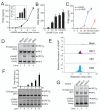
Figure 2. Purification and identification of the heat-resistant STING activator
(A) Full scan nano-LC-MS spectra of active and inactive fractions from the C18 column using LTQ. Arrows indicate an ion at +1 (675.11) and +2 (338.14) charge states present only in the active fraction. (B) Tandem mass (MS2) spectra after CID fragmentation of the ion with m/z =338.14 (z=2) from the MS1 scan shown in (A). Arrows indicate the m/z values of the expected fragmentation patterns of cyclic-GMP-AMP (cGAMP, bottom). Asterisk (*) indicates an ion (m/z=506) that resulted from a neutral loss of a water molecule (18) from the ion with m/z = 524. (C) Fractions (B7-B12) from the C18 column were analyzed for the presence of cGAMP by selective reaction monitoring of the expected ions and for their ability to stimulate IRF3 dimerization. (D) Comparison of the CID MS2 spectra of the purified STING activator and chemically synthesized cGAMP.
Figure 3. DNA transfection and DNA virus infection induce IFNβ through cGAMP
(A) Chemically synthesized cGAMP (100 nM) was delivered to digitonin-permeabilized L929 cells for indicated times, then IFNβ RNA and secreted protein were measured by q-RT-PCR (inset) and ELISA, respectively. Unless noted otherwise, the error bars in this and all other panels represent standard errors of the mean (n=3). (B) Similar to (A), except that different concentrations of cGAMP were delivered into L929 cells for 8 hr followed by q-RT-PCR analyses of IFNβ RNA. (C) Similar to (B), except that different concentrations of cGAMP and cdi-GMP were delivered into L929 cells followed by ELISA assays for IFNβ. (D) L929 cells were infected with HSV-1Δ34.5 or VSV-ΔM51-GFP, transfected with HT-DNA, or mock treated. An aliquot of the cell extracts was directly analyzed for IRF3 dimerization (top), whereas another aliquot was heated to denature proteins and the heat-resistant supernatant was assayed for its ability to stimulate IRF3 dimerization in permeabilized Raw264.7 cells (bottom). (E) The heat-resistant supernatant from (D) was fractionated by HPLC using a C18 column, and the presence of cGAMP in the fractions was measured by mass spectrometry using SRM. (F) L929 cells were transfected with 4 μg/ml HT-DNA for the indicated time, then IFNβ RNA was measured by q-RT-PCR and IRF3 dimerization was analyzed by native PAGE. Aliquots of the cell extracts were tested for the presence of cGAMP based on its ability to induce IRF3 dimerization after delivery into Raw264.7 cells. (G) THP1 cells were infected with HSV-1Δ34.5 and Vaccinia virus (VACV) for 6 hr, then the activation of endogenous IRF3 and generation of cGAMP activity were measured as described in (F).
Figure 4. cGAMP binds to STING and activates IRF3 in a STING-dependent manner
(A) Increasing concentrations of HT-DNA or cGAMP were delivered to indicated cells and the induction of IFNβ was measured by q-RT-PCR. Inset shows immunoblots of STING and β-tubulin in the cell lines. (B) Indicated cell lines were infected with HSV1Δ34.5 or permeabilized with digitionin and then incubated with cGAMP. Activation of endogenous IRF3 was analyzed by native gel electrophoresis (top). Aliquots of the cytosolic extracts were heated to denature proteins, and the supernatant was assayed for its ability to stimulate IRF3 in permeabilized Raw264.7 cells (bottom). (C) cGAMP, c-di-GMP, ISD, or poly[I:C] was delivered into L929 cells stably expressing a shRNA against GFP or STING for the indicated time, followed by analysis of IRF3 dimerization. (D) Recombinant STING protein was incubated with [32P]-ATP or [32P]-cGAMP in the presence or absence of the cold competitors as indicated. After UV crosslinking, the mixtures were resolved by SDS-PAGE followed by autoradiography.
Comment in
- Immunology. Sensing the dark side of DNA.
O'Neill LA. O'Neill LA. Science. 2013 Feb 15;339(6121):763-4. doi: 10.1126/science.1234724. Science. 2013. PMID: 23413341 No abstract available.
Similar articles
- Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway.
Sun L, Wu J, Du F, Chen X, Chen ZJ. Sun L, et al. Science. 2013 Feb 15;339(6121):786-91. doi: 10.1126/science.1232458. Epub 2012 Dec 20. Science. 2013. PMID: 23258413 Free PMC article. - Cyclic GMP-AMP as an Endogenous Second Messenger in Innate Immune Signaling by Cytosolic DNA.
Kato K, Omura H, Ishitani R, Nureki O. Kato K, et al. Annu Rev Biochem. 2017 Jun 20;86:541-566. doi: 10.1146/annurev-biochem-061516-044813. Epub 2017 Apr 7. Annu Rev Biochem. 2017. PMID: 28399655 Review. - Immunology. Sensing the dark side of DNA.
O'Neill LA. O'Neill LA. Science. 2013 Feb 15;339(6121):763-4. doi: 10.1126/science.1234724. Science. 2013. PMID: 23413341 No abstract available. - [Cytosolic DNA sensing by the cGAS-STING pathway in cancer].
Chanut R, Petrilli V. Chanut R, et al. Med Sci (Paris). 2019 Jun-Jul;35(6-7):527-534. doi: 10.1051/medsci/2019095. Epub 2019 Jul 5. Med Sci (Paris). 2019. PMID: 31274082 Review. French. - DNA sensing unchained.
Ablasser A, Hornung V. Ablasser A, et al. Cell Res. 2013 May;23(5):585-7. doi: 10.1038/cr.2013.28. Epub 2013 Feb 19. Cell Res. 2013. PMID: 23419517 Free PMC article.
Cited by
- Taking AIM at Influenza: The Role of the AIM2 Inflammasome.
Xu DW, Tate MD. Xu DW, et al. Viruses. 2024 Sep 27;16(10):1535. doi: 10.3390/v16101535. Viruses. 2024. PMID: 39459869 Free PMC article. Review. - Nucleic acid recognition orchestrates the anti-viral response to retroviruses.
Stavrou S, Blouch K, Kotla S, Bass A, Ross SR. Stavrou S, et al. Cell Host Microbe. 2015 Apr 8;17(4):478-88. doi: 10.1016/j.chom.2015.02.021. Epub 2015 Mar 26. Cell Host Microbe. 2015. PMID: 25816774 Free PMC article. - Nonstructural Protein A238L of the African Swine Fever Virus (ASFV) Enhances Antiviral Immune Responses by Activating the TBK1-IRF3 Pathway.
Liu W, Yang L, Di C, Sun J, Liu P, Liu H. Liu W, et al. Vet Sci. 2024 Jun 4;11(6):252. doi: 10.3390/vetsci11060252. Vet Sci. 2024. PMID: 38921999 Free PMC article. - Targeting the innate immune system as immunotherapy for acute myeloid leukemia.
Curran E, Corrales L, Kline J. Curran E, et al. Front Oncol. 2015 Apr 9;5:83. doi: 10.3389/fonc.2015.00083. eCollection 2015. Front Oncol. 2015. PMID: 25914882 Free PMC article. Review. - The role of cGAS in epithelial dysregulation in inflammatory bowel disease and gastrointestinal malignancies.
Ramos A, Bizri N, Novak E, Mollen K, Khan S. Ramos A, et al. Front Pharmacol. 2024 Jul 10;15:1409683. doi: 10.3389/fphar.2024.1409683. eCollection 2024. Front Pharmacol. 2024. PMID: 39050748 Free PMC article. Review.
References
- Barbalat R, Ewald SE, Mouchess ML, Barton GM. Nucleic Acid Recognition by the Innate Immune System. Annu Rev Immunol. Apr 5 - PubMed
- Barber GN. Cytoplasmic DNA innate immune pathways. Immunological reviews. 2011 Sep;243:99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI-093967/AI/NIAID NIH HHS/United States
- R01 GM079554/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 AI093967/AI/NIAID NIH HHS/United States
- GM-079554/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials