Diabetes-related adduct formation and retinopathy - PubMed (original) (raw)
Diabetes-related adduct formation and retinopathy
Alan W Stitt et al. J Ocul Biol Dis Infor. 2011 Jun.
Abstract
The pathogenesis of diabetic retinopathy is complex, reflecting the array of systemic and tissue-specific metabolic abnormalities. A range of pathogenic pathways are directly linked to hyperglycaemia and dyslipidaemia, and the retina appears to be exquisitely sensitive to damage. Establishing the biochemical and molecular basis for this pathology remains an important research focus. This review concentrates on the formation of a range of protein adducts that form after exposure to modifying intermediates known to be elevated during diabetes. These so-called advanced glycation end products (AGEs) and advanced lipoxidation end products (ALEs) are thought to play an important role in the initiation and progression of diabetic retinopathy, and mechanisms leading to dysfunction and death of various retinal cells are becoming understood. Perspective is provided on AGE/ALE formation in the retina and the impact that such adducts have on retinal cell function. There will be emphasis placed on the role of the receptor for AGEs and how this may modulate retinal pathology, especially in relation to oxidative stress and inflammation. The review will conclude by discussion of strategies to inhibit AGE/ALE formation or harmful receptor interactions in order to prevent disease progression from the point of diabetes diagnosis to sight-threatening proliferative diabetic retinopathy and diabetic macular oedema.
Keywords: Advanced glycation; Diabetic retinopathy; Lipoxidation.
Figures
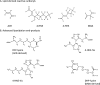
Fig. 1
The structures of lipoxidation-derived reactive carbonyl species and the principal advanced lipoxidation adducts formed following their reaction with proteins
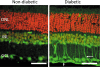
Fig. 2
FDP-lysine immunoreactivity (green channel) in retinal sections from non-diabetic and diabetic rats of 4-month disease duration. Cell nuclei are stained red by propidium iodide. In diabetes, prominent immunolabelling appeared in Müller glia end-feet and radial processes (arrows). GCL ganglion cell layer, INL inner nuclear layer, ONL outer nuclear layer. Scale bars = 50μm
Fig. 3
Phase-contrast micrographs of cultured human MIO-M1 Müller glia exposed to increasing concentrations of FDP-lysine-modified human serum albumin (FDP-lysine-HSA). High concentrations of FDP-lysine-HSA (0.5 mg/mL) induce cell death. Scale bars = 100μm
Similar articles
- Advanced glycation and advanced lipoxidation: possible role in initiation and progression of diabetic retinopathy.
Stitt AW, Frizzell N, Thorpe SR. Stitt AW, et al. Curr Pharm Des. 2004;10(27):3349-60. doi: 10.2174/1381612043383124. Curr Pharm Des. 2004. PMID: 15544520 Review. - AGEs and diabetic retinopathy.
Stitt AW. Stitt AW. Invest Ophthalmol Vis Sci. 2010 Oct;51(10):4867-74. doi: 10.1167/iovs.10-5881. Invest Ophthalmol Vis Sci. 2010. PMID: 20876889 Review. - Müller glial dysfunction during diabetic retinopathy in rats is linked to accumulation of advanced glycation end-products and advanced lipoxidation end-products.
Curtis TM, Hamilton R, Yong PH, McVicar CM, Berner A, Pringle R, Uchida K, Nagai R, Brockbank S, Stitt AW. Curtis TM, et al. Diabetologia. 2011 Mar;54(3):690-8. doi: 10.1007/s00125-010-1971-x. Epub 2010 Nov 30. Diabetologia. 2011. PMID: 21116609 - Advanced glycation end products and diabetic retinopathy.
Chen M, Curtis TM, Stitt AW. Chen M, et al. Curr Med Chem. 2013;20(26):3234-40. doi: 10.2174/09298673113209990025. Curr Med Chem. 2013. PMID: 23745547 Review. - The role of advanced glycation in the pathogenesis of diabetic retinopathy.
Stitt AW. Stitt AW. Exp Mol Pathol. 2003 Aug;75(1):95-108. doi: 10.1016/s0014-4800(03)00035-2. Exp Mol Pathol. 2003. PMID: 12834631 Review.
Cited by
- Primary retinal cultures as a tool for modeling diabetic retinopathy: an overview.
Matteucci A, Varano M, Mallozzi C, Gaddini L, Villa M, Gabrielli S, Formisano G, Pricci F, Malchiodi-Albedi F. Matteucci A, et al. Biomed Res Int. 2015;2015:364924. doi: 10.1155/2015/364924. Epub 2015 Jan 19. Biomed Res Int. 2015. PMID: 25688355 Free PMC article. Review. - Advanced glycation end products and diabetic complications.
Singh VP, Bali A, Singh N, Jaggi AS. Singh VP, et al. Korean J Physiol Pharmacol. 2014 Feb;18(1):1-14. doi: 10.4196/kjpp.2014.18.1.1. Epub 2014 Feb 13. Korean J Physiol Pharmacol. 2014. PMID: 24634591 Free PMC article. Review. - Prevention of protein glycation by natural compounds.
Sadowska-Bartosz I, Bartosz G. Sadowska-Bartosz I, et al. Molecules. 2015 Feb 16;20(2):3309-34. doi: 10.3390/molecules20023309. Molecules. 2015. PMID: 25690291 Free PMC article. Review. - A review on mechanism of inhibition of advanced glycation end products formation by plant derived polyphenolic compounds.
Anwar S, Khan S, Almatroudi A, Khan AA, Alsahli MA, Almatroodi SA, Rahmani AH. Anwar S, et al. Mol Biol Rep. 2021 Jan;48(1):787-805. doi: 10.1007/s11033-020-06084-0. Epub 2021 Jan 3. Mol Biol Rep. 2021. PMID: 33389535 Review. - Role of Glycation in Type 2 Diabetes Mellitus and Its Prevention through Nymphaea Species.
Ishrat N, Khan H, Patel OPS, Mahdi AA, Mujeeb F, Ahmad S. Ishrat N, et al. Biomed Res Int. 2021 Oct 27;2021:7240046. doi: 10.1155/2021/7240046. eCollection 2021. Biomed Res Int. 2021. PMID: 34746307 Free PMC article. Review.
References
- Aiello LP, Gardner TW, King GL, et al. Diabetic retinopathy. Diabetes Care. 1998;21:143–156. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources