Dose-dependent effects of vitamin D on transdifferentiation of skeletal muscle cells to adipose cells - PubMed (original) (raw)
Dose-dependent effects of vitamin D on transdifferentiation of skeletal muscle cells to adipose cells
Kevin J P Ryan et al. J Endocrinol. 2013.
Abstract
Fat infiltration within muscle is one of a number of features of vitamin D deficiency, which leads to a decline in muscle functionality. The origin of this fat is unclear, but one possibility is that it forms from myogenic precursor cells present in the muscle, which transdifferentiate into mature adipocytes. The current study examined the effect of the active form of vitamin D₃, 1,25-dihydroxyvitamin D₃ (1,25(OH)₂D₃), on the capacity of the C2C12 muscle cell line to differentiate towards the myogenic and adipogenic lineages. Cells were cultured in myogenic or adipogenic differentiation media containing increasing concentrations (0, 10⁻¹³, 10⁻¹¹, 10⁻⁹, 10⁻⁷ or 10⁻⁵ M) of 1,25(OH)₂D₃ for up to 6 days and markers of muscle and fat development measured. Mature myofibres were formed in both adipogenic and myogenic media, but fat droplets were only observed in adipogenic media. Relative to controls, low physiological concentrations (10⁻¹³ and 10⁻¹¹ M) of 1,25(OH)₂D3 increased fat droplet accumulation, whereas high physiological (10⁻⁹ M) and supraphysiological concentrations (≥10⁻⁷ M) inhibited fat accumulation. This increased accumulation of fat with low physiological concentrations (10⁻¹³ and 10⁻¹¹ M) was associated with a sequential up-regulation of PPARγ2 (PPARG) and FABP4 mRNA, indicating formation of adipocytes, whereas higher concentrations (≥10⁻⁹ M) reduced all these effects, and the highest concentration (10⁻⁵ M) appeared to have toxic effects. This is the first study to demonstrate dose-dependent effects of 1,25(OH)₂D₃ on the transdifferentiation of muscle cells into adipose cells. Low physiological concentrations (possibly mimicking a deficient state) induced adipogenesis, whereas higher (physiological and supraphysiological) concentrations attenuated this effect.
Figures
Figure 1
Dose-dependent effects of 1,25(OH)2D3 on accumulation of lipid droplets in C2C12 cells cultured in myogenic (A, B, C, D, E and F) or adipogenic (G, H, I, J, K and L) media. Representative images of C2C12 cells stained with Oil Red-O (as indicated by white arrows) and counterstained with haematoxylin to indicate lipid droplet and nuclei/myofibre structures respectively. Cells were cultured for 6 days in myogenic (A, B, C, D, E and F) or adipogenic (G, H, I, J, K and L) media with vehicle (A and G) or increasing concentrations of 1,25(OH)2D3 (10−13 M (B and H), 10−11 M (C and I), 10−9 M (D and J), 10−7 M (E and K) or 10−5 M (F and L)).
Figure 2
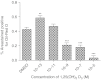
Dose-dependent effects of 1,25(OH)2D3 on percentage area of lipid accumulation in C2C12 cells cultured in adipogenic media for 6 days. C2C12 cells were stained with Oil Red-O and counterstained with haematoxylin, before the percentage area of the red stain within a field of view (FOV) was quantified by image analysis to give a representative percentage area per well (_n_=4 wells, five FOV/well). One-way ANOVA indicated a significant effect of 1,25(OH)2D3 (P<0.001). Significant differences compared to DMSO control cells were determined by post-hoc Bonferroni's test (**P<0.01 and ***P<0.001).
Figure 3
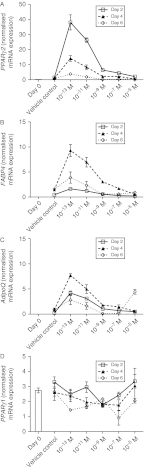
Dose-dependent effects of 1,25(OH)2D3 on expression of white adipocyte marker genes. Expression of white adipocyte marker genes was determined by quantitative RT-PCR analysis. Levels of (A) Ppar γ 2, (B) Fabp4, (C) Adipoq/adiponectin and (D) Ppar γ 1 mRNAs were quantified in C2C12 cells cultured in the absence or presence of 10−13, 10−11, 10−9, 10−7 or 10−5 M 1,25(OH)2D3 for 2, 4 or 6 days in adipogenic differentiation media. Expression at day 0 (before differentiation media and 1,25(OH)2D3 was added) is also included and is indicated by a bar (in some instances, this was very low). Significant two-way interactions between day of differentiation and 1,25(OH)2D3 concentration were observed for PPARγ2, FABP4 and AdipoQ (P<0.001 for all). For PPARγ1, there was a significant effect of stage of differentiation (_P_=0.002) and a significant effect of 1,25(OH)2D3 concentration (_P_=0.005), but no interaction.
Figure 4
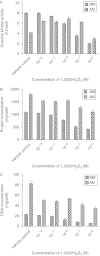
Dose-dependent effects of 1,25(OH)2D3 on (A) CK activity (IU/well), (B) protein (μg/well) and (C) DNA content (μg/well) in C2C12 cells cultured for 4 days in myogenic media (MM) or adipogenic media (AM) with increasing concentrations (vehicle control, 10−13, 10−11, 10−9, 10−7 and 10−5 M) of 1,25(OH)2D3. Significant two-way interactions between media type and 1,25(OH)2D3 concentration were observed for CK activity (P<0.001), protein (P<0.01) and DNA (P<0.001) content.
Figure 5
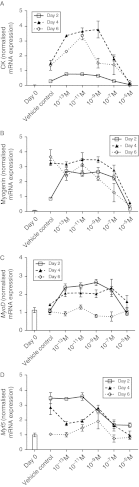
Dose-dependent effects of 1,25(OH)2D3 on expression of skeletal muscle marker genes. Expression of skeletal muscle marker genes was determined by quantitative RT-PCR analysis. Levels of (A) CK, (B) myogenin, (C) MyoD and (D) Myf5 mRNAs were quantified in C2C12 cells cultured in the absence or presence of 10−13, 10−11, 10−9, 10−7 or 10−5 M 1,25(OH)2D3 for 2, 4 or 6 days in adipogenic differentiation media. Expression at day 0 (before differentiation media and 1,25(OH)2D3 was added) is also included for reference and is indicated by a bar. Significant two-way interactions between day of differentiation and 1,25(OH)2D3 concentration were observed for CK, myogenin, Myf5 (P<0.001 for all) and MyoD (P<0.05) mRNA transcripts.
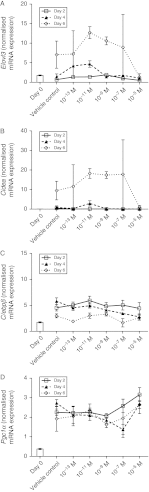
Figure 6
Dose-dependent effects of 1,25(OH)2D3 on expression of brown adipocyte marker genes. Expression of brown adipocyte marker genes was determined by quantitative RT-PCR analysis. Levels of (A) Elovl3, (B) Cidea, (C) C/ebp β and (D) Pgc1 α mRNAs were quantified in C2C12 cells cultured in the absence or presence of 10−13, 10−11, 10−9, 10−7 or 10−5 M 1,25(OH)2D3 for 2, 4 or 6 days in adipogenic differentiation media. Expression at day 0 (before differentiation media and 1,25(OH)2D3 was added) is also included for reference and is indicated by a bar. Significant effects of day of differentiation were observed for Elovl3, Cidea and C/ebpβ (P<0.001 for all three), but not PGC1α (_P_>0.05). There were no significant effects of 1,25(OH)2D3 concentration on any of the brown adipocyte marker genes.
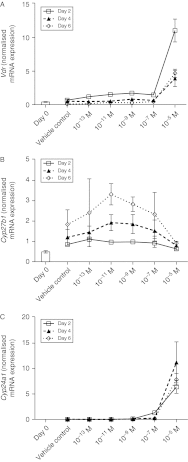
Figure 7
Dose-dependent effects of 1,25(OH)2D3 on expression of VDR, CYP27B1 (1α-hydroxylase) and CYP24A1 (24-hydroxylase) enzymes. Expression of VitD-related genes was determined by quantitative RT-PCR analysis. Levels of (A) Vdr, (B) Cyp27b1 and (C) Cyp24a1 mRNAs were quantified in C2C12 cells cultured in the absence or presence of 10−13, 10−11, 10−9, 10−7 or 10−5 M 1,25(OH)2D3 for 2, 4 or 6 days in adipogenic differentiation media. Expression at day 0 (before differentiation media and 1,25(OH)2D3 was added) is also included for reference and is indicated by a bar. A significant two-way interaction (P<0.001) between day of differentiation and 1,25(OH)2D3 concentration was observed for VDR only. There was a significant effect of day of differentiation (_P_=0.001) on expression of Cyp27b1 mRNA, but no effect of 1,25(OH)2D3 concentration. By contrast, there was a significant effect (P<0.001) of 1,25(OH)2D3 concentration on Cyp24a1 mRNA, but no effect of day of differentiation.
Similar articles
- Human fetal mesenchymal stem cells differentiate into brown and white adipocytes: a role for ERRalpha in human UCP1 expression.
Morganstein DL, Wu P, Mane MR, Fisk NM, White R, Parker MG. Morganstein DL, et al. Cell Res. 2010 Apr;20(4):434-44. doi: 10.1038/cr.2010.11. Epub 2010 Jan 26. Cell Res. 2010. PMID: 20101261 Free PMC article. - PRDM16 controls a brown fat/skeletal muscle switch.
Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. Seale P, et al. Nature. 2008 Aug 21;454(7207):961-7. doi: 10.1038/nature07182. Nature. 2008. PMID: 18719582 Free PMC article. - BMP4 and BMP7 induce the white-to-brown transition of primary human adipose stem cells.
Elsen M, Raschke S, Tennagels N, Schwahn U, Jelenik T, Roden M, Romacho T, Eckel J. Elsen M, et al. Am J Physiol Cell Physiol. 2014 Mar 1;306(5):C431-40. doi: 10.1152/ajpcell.00290.2013. Epub 2013 Nov 27. Am J Physiol Cell Physiol. 2014. PMID: 24284793 - Nutritional factors in transdifferentiation of scheletal muscles to adipocytes.
Pînzariu A, Sindilar A, Haliga R, Chelaru L, Mocanu V. Pînzariu A, et al. Rev Med Chir Soc Med Nat Iasi. 2014 Jul-Sep;118(3):699-705. Rev Med Chir Soc Med Nat Iasi. 2014. PMID: 25341288 Review. - Factors inducing transdifferentiation of myoblasts into adipocytes.
Wang L, Shan T. Wang L, et al. J Cell Physiol. 2021 Apr;236(4):2276-2289. doi: 10.1002/jcp.30074. Epub 2020 Sep 28. J Cell Physiol. 2021. PMID: 32989814 Review.
Cited by
- Resveratrol and Vitamin D: Eclectic Molecules Promoting Mitochondrial Health in Sarcopenia.
Russo C, Valle MS, D'Angeli F, Surdo S, Malaguarnera L. Russo C, et al. Int J Mol Sci. 2024 Jul 9;25(14):7503. doi: 10.3390/ijms25147503. Int J Mol Sci. 2024. PMID: 39062745 Free PMC article. Review. - Differential effects of cholecalciferol and calcitriol on muscle proteolysis and oxidative stress in angiotensin II-induced C2C12 myotube atrophy.
Hirunsai M, Srikuea R. Hirunsai M, et al. Physiol Rep. 2024 Apr;12(8):e16011. doi: 10.14814/phy2.16011. Physiol Rep. 2024. PMID: 38627219 Free PMC article. - Active vitamin D increases myogenic differentiation in C2C12 cells via a vitamin D response element on the myogenin promoter.
Alliband KH, Parr T, Jethwa PH, Brameld JM. Alliband KH, et al. Front Physiol. 2024 Jan 8;14:1322677. doi: 10.3389/fphys.2023.1322677. eCollection 2023. Front Physiol. 2024. PMID: 38264331 Free PMC article. - Intermuscular adipose tissue in obesity and related disorders: cellular origins, biological characteristics and regulatory mechanisms.
Zhang T, Li J, Li X, Liu Y. Zhang T, et al. Front Endocrinol (Lausanne). 2023 Oct 18;14:1280853. doi: 10.3389/fendo.2023.1280853. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37920255 Free PMC article. Review. - Targeting Crosstalk of Signaling Pathways among Muscles-Bone-Adipose Tissue: A Promising Therapeutic Approach for Sarcopenia.
Sharma AR, Chatterjee S, Lee YH, Lee SS. Sharma AR, et al. Aging Dis. 2024 Aug 1;15(4):1619-1645. doi: 10.14336/AD.2023.00903. Aging Dis. 2024. PMID: 37815907 Free PMC article. Review.
References
- Bischoff HA, Stahelin HB, Dick W, Akos R, Knecht M, Salis C, Nebiker M, Theiler R, Pfeifer M, Begerow B, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. Journal of Bone and Mineral Research. 2003;18:343–351. doi: 10.1359/jbmr.2003.18.2.343. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials