Discoidin domain receptors: unique receptor tyrosine kinases in collagen-mediated signaling - PubMed (original) (raw)
Review
Discoidin domain receptors: unique receptor tyrosine kinases in collagen-mediated signaling
Hsueh-Liang Fu et al. J Biol Chem. 2013.
Abstract
The discoidin domain receptors (DDRs) are receptor tyrosine kinases that recognize collagens as their ligands. DDRs display unique structural features and distinctive activation kinetics, which set them apart from other members of the kinase superfamily. DDRs regulate cell-collagen interactions in normal and pathological conditions and thus are emerging as major sensors of collagen matrices and potential novel therapeutic targets. New structural and biological information has shed light on the molecular mechanisms that regulate DDR signaling, turnover, and function. This minireview provides an overview of these areas of DDR research with the goal of fostering further investigation of these intriguing and unique receptors.
Figures
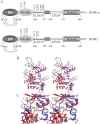
FIGURE 1.
A, domain structure of DDRs. Only cysteine residues involved in intramolecular disulfide bond formation are shown. Predicted _N_-glycosylation (italic) and _O_-glycosylation (underlined) sites are indicated. B, ribbon representation of the modeled DFG-in (red) and DFG-out (blue) conformations of the DDR1a KD shown in stereo. C, close-up stereoview of the catalytic pocket with several key residues in stick representation.
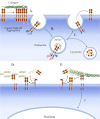
FIGURE 2.
DDR1 endocytosis and shedding. Activation of DDR1 dimers by collagen induces receptor aggregation (A) and triggers endocytosis of the receptor-ligand complex into Rab5-positive endosomes (B), which can lead to lysosomal degradation or recycling of the receptor to the cell surface (C). The presence of the NP_X_Y motif within the IJXM region of the DDR1b and DDR1c isoforms may confer on these receptors a unique endocytic trafficking route by mediating association with specific adaptor/scaffold proteins (pink and blue shapes). Within the endosomal compartment, DDR1 may display continued signaling. DDR1 is cleaved by metalloproteinases, including MT-MMPs and ADAMs, at the cell surface, releasing the ectodomain and generating a C-terminal fragment (D and E). Together with the full-length receptor, the released DDR1 ectodomain can play a role in regulation of collagen fibrillogenesis (D) and or block ligand binding (E). The action of MT-MMPs on collagen may also regulate DDR1 signaling by altering the integrity of the collagen matrix (D and E). The C-terminal fragment of DDR1 may translocate to the nucleus (E). At present, there is no information on DDR2 internalization and shedding.
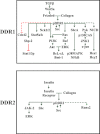
FIGURE 3.
DDR signaling. The wiring diagram depicts signaling effectors of DDR activated in response to collagen. Solid and dashed green arrows indicate direct and indirect positive signaling effectors upstream and downstream of phosphorylated DDRs (pDDR), respectively. Red lines indicate direct (solid) and indirect (dashed) negative effects of phosphorylated DDRs on signaling effectors.
Similar articles
- Collagen recognition and transmembrane signalling by discoidin domain receptors.
Carafoli F, Hohenester E. Carafoli F, et al. Biochim Biophys Acta. 2013 Oct;1834(10):2187-94. doi: 10.1016/j.bbapap.2012.10.014. Epub 2012 Nov 2. Biochim Biophys Acta. 2013. PMID: 23128141 Free PMC article. Review. - Discoidin domain receptor functions in physiological and pathological conditions.
Leitinger B. Leitinger B. Int Rev Cell Mol Biol. 2014;310:39-87. doi: 10.1016/B978-0-12-800180-6.00002-5. Int Rev Cell Mol Biol. 2014. PMID: 24725424 Free PMC article. Review. - Discoidin domain receptor tyrosine kinases: new players in cancer progression.
Valiathan RR, Marco M, Leitinger B, Kleer CG, Fridman R. Valiathan RR, et al. Cancer Metastasis Rev. 2012 Jun;31(1-2):295-321. doi: 10.1007/s10555-012-9346-z. Cancer Metastasis Rev. 2012. PMID: 22366781 Free PMC article. Review. - Sensing extracellular matrix: an update on discoidin domain receptor function.
Vogel WF, Abdulhussein R, Ford CE. Vogel WF, et al. Cell Signal. 2006 Aug;18(8):1108-16. doi: 10.1016/j.cellsig.2006.02.012. Epub 2006 Feb 28. Cell Signal. 2006. PMID: 16626936 Review. - Molecular analysis of collagen binding by the human discoidin domain receptors, DDR1 and DDR2. Identification of collagen binding sites in DDR2.
Leitinger B. Leitinger B. J Biol Chem. 2003 May 9;278(19):16761-9. doi: 10.1074/jbc.M301370200. Epub 2003 Feb 28. J Biol Chem. 2003. PMID: 12611880
Cited by
- Cryptic collagen elements as signaling hubs in the regulation of tumor growth and metastasis.
Han X, Caron JM, Brooks PC. Han X, et al. J Cell Physiol. 2020 Dec;235(12):9005-9020. doi: 10.1002/jcp.29752. Epub 2020 May 12. J Cell Physiol. 2020. PMID: 32400053 Free PMC article. Review. - Inhibition of SYK and cSrc kinases can protect bone and cartilage in preclinical models of osteoarthritis and rheumatoid arthritis.
Novikov FN, Panova MV, Titov IY, Stroylov VS, Stroganov OV, Chilov GG. Novikov FN, et al. Sci Rep. 2021 Nov 30;11(1):23120. doi: 10.1038/s41598-021-02568-6. Sci Rep. 2021. PMID: 34848799 Free PMC article. - Reciprocal discoidin domain receptor signaling strengthens integrin adhesion to connect adjacent tissues.
Park K, Jayadev R, Payne SG, Kenny-Ganzert IW, Chi Q, Costa DS, Ramos-Lewis W, Thendral SB, Sherwood DR. Park K, et al. bioRxiv [Preprint]. 2023 May 16:2023.03.14.532639. doi: 10.1101/2023.03.14.532639. bioRxiv. 2023. PMID: 36993349 Free PMC article. Updated. Preprint. - Tissue Force Programs Cell Fate and Tumor Aggression.
Northey JJ, Przybyla L, Weaver VM. Northey JJ, et al. Cancer Discov. 2017 Nov;7(11):1224-1237. doi: 10.1158/2159-8290.CD-16-0733. Epub 2017 Oct 16. Cancer Discov. 2017. PMID: 29038232 Free PMC article. Review. - Two-step release of kinase autoinhibition in discoidin domain receptor 1.
Sammon D, Hohenester E, Leitinger B. Sammon D, et al. Proc Natl Acad Sci U S A. 2020 Sep 8;117(36):22051-22060. doi: 10.1073/pnas.2007271117. Epub 2020 Aug 24. Proc Natl Acad Sci U S A. 2020. PMID: 32839343 Free PMC article.
References
- Di Marco E., Cutuli N., Guerra L., Cancedda R., De Luca M. (1993) Molecular cloning of trkE, a novel trk-related putative tyrosine kinase receptor isolated from normal human keratinocytes and widely expressed by normal human tissues. J. Biol. Chem. 268, 24290–24295 - PubMed
- Zerlin M., Julius M. A., Goldfarb M. (1993) NEP: a novel receptor-like tyrosine kinase expressed in proliferating neuroepithelia. Oncogene 8, 2731–2739 - PubMed
- Perez J. L., Jing S. Q., Wong T. W. (1996) Identification of two isoforms of the Cak receptor kinase that are coexpressed in breast tumor cell lines. Oncogene 12, 1469–1477 - PubMed
- Laval S., Butler R., Shelling A. N., Hanby A. M., Poulsom R., Ganesan T. S. (1994) Isolation and characterization of an epithelial-specific receptor tyrosine kinase from an ovarian cancer cell line. Cell Growth Differ. 5, 1173–1183 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources