β-Cell failure in type 2 diabetes: a case of asking too much of too few? - PubMed (original) (raw)
β-Cell failure in type 2 diabetes: a case of asking too much of too few?
Safia Costes et al. Diabetes. 2013 Feb.
Abstract
The islet in type 2 diabetes (T2DM) is characterized by a deficit in β-cells, increased β-cell apoptosis, and extracellular amyloid deposits derived from islet amyloid polypeptide (IAPP). In the absence of longitudinal studies, it is unknown if the low β-cell mass in T2DM precedes diabetes onset (is a risk factor for diabetes) or develops as a consequence of the disease process. Although insulin resistance is a risk factor for T2DM, most individuals who are insulin resistant do not develop diabetes. By inference, an increased β-cell workload results in T2DM in some but not all individuals. We propose that the extent of the β-cell mass that develops during childhood may underlie subsequent successful or failed adaptation to insulin resistance in later life. We propose that a low innate β-cell mass in the face of subsequent insulin resistance may expose β-cells to a burden of insulin and IAPP biosynthetic demand that exceeds the cellular capacity for protein folding and trafficking. If this threshold is crossed, intracellular toxic IAPP membrane permeant oligomers (cylindrins) may form, compromising β-cell function and inducing β-cell apoptosis.
Figures
FIG. 1.
A: Human islets from a nondiabetic subject and a subject with T2DM (upper panel) and from a wild-type (WT) and a human IAPP transgenic (HIP) rat (lower panel) stained for insulin (brown). Deposits of amyloid derived from IAPP are indicated by a white arrowhead. Original magnification: ×40. B: Alignment of IAPP ortholog proteins. Amino acid alignment of IAPP protein sequences identified in Homo sapiens (human, CAA39504), human mutant (S20G), Macaca mulatto (monkey, XP_001098290), Felis catus (cat, NP_001036803), Mus musculus (mouse, NP_034621), and Rattus norvegicus (rat, NP_036718). Dots correspond to conserved residues with human IAPP sequence. Red letters correspond to the amyloidogenic sequence. C: Sections of islets from human IAPP transgenic mice labeled for oligomers (A11) and IAPP (5 nm and 10 nm gold, respectively). IAPP- and oligomer-labeled aggregates were found adjacent to mitochondria (M), and mitochondrial integrity appeared to be compromised (black arrow points to the aggregates penetrating mitochondria). Original magnification: ×120,000. This figure originally appeared in an article by Gurlo et al. (50).
FIG. 2.
IAPP misfolding pathways. A: Schematic illustration of a stepwise misfolding pathway of IAPP that generates toxic oligomers as well as a range of different fibril types. Although the structure of IAPP oligomers remains elusive, the crystal structure of an A11 antibody–positive oligomer structure has recently been reported (25) and is shown in panel B. C and D: The reported structures for fibrils with striated ribbons and twisted morphologies. The bottom shows the structure of single IAPP molecule (green). In case of the striated ribbon, the two strands are approximately in the same plane while the two strands in the fibrils with twisted morphology are offset. Individual stacks of monomers were built using MFIBRIL (
http://chemsoft.hsc.usc.edu:8080/MFIBRIL/
) and colored green, brown, and blue. MFIBRIL was then used to dock individual stacks together to better mimic the fibril structure (blue and magenta). Note that contacts in the striated ribbons are made between same monomeric subunits, whereas contacts in the twisted fibrils are more staggered by packing strands from one monomer subunit against strands from a monomer three layers above.
FIG. 3.
Secretory pathway and mechanisms of β-cell defense against protein misfolding. The major β-cell secretory proteins, insulin and IAPP, are synthesized and folded in the ER and then processed within the secretory pathway (Golgi and secretory vesicles). Misfolded proteins are targeted to the ER-associated degradation, also known as ubiquitin-proteasome system (UPS), that involves ubiquitination of the targeted proteins, their deubiquitination by enzymes such as UCH-L1, and subsequent degradation by the proteasome. If the ubiquitin-proteasome system fails or if protein aggregates form, an alternative pathway of protein clearance becomes available: the autophagy pathway in which membranes surround the material to be degraded (ubiquitinated proteins and protein aggregates but also damaged organelles and aged vesicles) to form autophagosomes that fuse with lysosomes to allow degradation of their content.
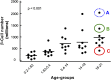
FIG. 4.
β-Cell mass growth varies widely in childhood. Postnatal expansion of β-cell number plays a major role in establishing β-cell mass in adult humans and is highly variable between individuals. Data are from Meier et al. (60). Total number of β-cells in 46 children aged 2 weeks to 21 years. Data are represented as individual data points. Individuals with high (A, blue), intermediate (B, green), and low (C, red) β-cell numbers are shown for consideration of β-cell workload in response to obesity in Fig. 5.
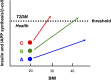
FIG. 5.
Interaction of postnatal β-cell mass and BMI on insulin and IAPP synthetic demand. Schematic representation of the risk of T2DM in individuals with high (A, blue), intermediate (B, green), and low (C, red) β-cell mass formed after postnatal growth (see Fig. 4) with consideration of their BMI. The increment in the protein synthetic burden per β-cell increases more steeply in those with low (individual C) versus a high number of β-cells (individual A). The burden placed on β-cells by obesity is thus higher in individual C, as is the risk to breach the threshold for protein folding and disposal, ultimately leading to β-cell failure in T2DM.
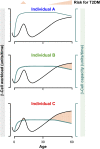
FIG. 6.
β-Cell workload and risk of T2DM. Schematic representation of β-cell workload (in black) and β-cell work capacity (green) throughout life. β-Cell workload increases transiently during adolescence and progressively with aging. The capacity for β-cell workload is defined by the β-cell mass after the postnatal expansion (high in individual A, intermediate in individual B, and low in individual C, see Fig. 4) and β-cell ability to defend against protein misfolding (declines in all individuals with aging). T2DM risk increases when workload exceeds capacity (light orange), in adolescence and early adult life in C, later in B, and only with advanced age in A.
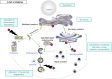
FIG. 7.
Consequences of formation of intracellular toxic IAPP oligomers (cylindrins) in T2DM. Toxic IAPP oligomers (in red) are formed intracellularly in β-cells and escape from the secretory pathway leading to intracellular membrane disruption (ER, Golgi, vesicles, mitochondria), ER stress, alteration of proteasomal degradation through deficit in UCH-L1, and alteration of the autophagy/lysosomal degradation, ultimately leading to β-cell failure and apoptosis (49,50,53,58).
Similar articles
- Successful versus failed adaptation to high-fat diet-induced insulin resistance: the role of IAPP-induced beta-cell endoplasmic reticulum stress.
Matveyenko AV, Gurlo T, Daval M, Butler AE, Butler PC. Matveyenko AV, et al. Diabetes. 2009 Apr;58(4):906-16. doi: 10.2337/db08-1464. Epub 2009 Jan 16. Diabetes. 2009. PMID: 19151199 Free PMC article. - Human IAPP amyloidogenic properties and pancreatic β-cell death.
Fernández MS. Fernández MS. Cell Calcium. 2014 Nov;56(5):416-27. doi: 10.1016/j.ceca.2014.08.011. Epub 2014 Aug 27. Cell Calcium. 2014. PMID: 25224501 Review. - UCHL1 deficiency exacerbates human islet amyloid polypeptide toxicity in β-cells: evidence of interplay between the ubiquitin/proteasome system and autophagy.
Costes S, Gurlo T, Rivera JF, Butler PC. Costes S, et al. Autophagy. 2014 Jun;10(6):1004-14. doi: 10.4161/auto.28478. Autophagy. 2014. PMID: 24879150 Free PMC article. - Evidence for proteotoxicity in beta cells in type 2 diabetes: toxic islet amyloid polypeptide oligomers form intracellularly in the secretory pathway.
Gurlo T, Ryazantsev S, Huang CJ, Yeh MW, Reber HA, Hines OJ, O'Brien TD, Glabe CG, Butler PC. Gurlo T, et al. Am J Pathol. 2010 Feb;176(2):861-9. doi: 10.2353/ajpath.2010.090532. Epub 2009 Dec 30. Am J Pathol. 2010. PMID: 20042670 Free PMC article. - Causative factors for formation of toxic islet amyloid polypeptide oligomer in type 2 diabetes mellitus.
Jeong HR, An SS. Jeong HR, et al. Clin Interv Aging. 2015 Nov 19;10:1873-9. doi: 10.2147/CIA.S95297. eCollection 2015. Clin Interv Aging. 2015. PMID: 26604727 Free PMC article. Review.
Cited by
- Core circadian transcription factor Bmal1 mediates β cell response and recovery from pro-inflammatory injury.
Rakshit K, Brown MR, Javeed N, Lee JH, Ordog T, Matveyenko AV. Rakshit K, et al. iScience. 2024 Oct 16;27(11):111179. doi: 10.1016/j.isci.2024.111179. eCollection 2024 Nov 15. iScience. 2024. PMID: 39524327 Free PMC article. - The Counteracting Effects of Ang II and Ang-(1-7) on the Function andGrowth of Insulin-secreting NIT-1 Cells.
Lin X, Wang X, Feng W, Wan Y, Chai J, Li F, Xu M. Lin X, et al. Curr Diabetes Rev. 2024;20(10):e010124225112. doi: 10.2174/0115733998276291231204115314. Curr Diabetes Rev. 2024. PMID: 38173074 - Interaction between Autophagy and Senescence in Pancreatic Beta Cells.
Hela F, Aguayo-Mazzucato C. Hela F, et al. Biology (Basel). 2023 Sep 4;12(9):1205. doi: 10.3390/biology12091205. Biology (Basel). 2023. PMID: 37759604 Free PMC article. Review. - Differentially Expressed Genes Regulating Glutathione Metabolism, Protein-Folding, and Unfolded Protein Response in Pancreatic β-Cells in Type 2 Diabetes Mellitus.
Klyosova E, Azarova I, Buikin S, Polonikov A. Klyosova E, et al. Int J Mol Sci. 2023 Jul 27;24(15):12059. doi: 10.3390/ijms241512059. Int J Mol Sci. 2023. PMID: 37569434 Free PMC article. - Effects of acute changes in fasting glucose and free fatty acid concentrations on indices of β-cell function and glucose metabolism in subjects without diabetes.
Schembri Wismayer D, Laurenti MC, Song Y, Egan AM, Welch AA, Bailey KR, Cobelli C, Dalla Man C, Jensen MD, Vella A. Schembri Wismayer D, et al. Am J Physiol Endocrinol Metab. 2023 Aug 1;325(2):E119-E131. doi: 10.1152/ajpendo.00043.2023. Epub 2023 Jun 7. Am J Physiol Endocrinol Metab. 2023. PMID: 37285600 Free PMC article.
References
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 - PubMed
- Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 2008;10(Suppl. 4):32–42 - PubMed
- Klöppel G, Löhr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res 1985;4:110–125 - PubMed
- Clark A, Cooper GJ, Lewis CE, et al. Islet amyloid formed from diabetes-associated peptide may be pathogenic in type-2 diabetes. Lancet 1987;2:231–234 - PubMed
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 2002;297:353–356 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK061539/DK/NIDDK NIH HHS/United States
- R01 DK061539/DK/NIDDK NIH HHS/United States
- R01 AG027936/AG/NIA NIH HHS/United States
- R01 DK077967/DK/NIDDK NIH HHS/United States
- AG027936/AG/NIA NIH HHS/United States
- R01 DK059579/DK/NIDDK NIH HHS/United States
- DK059579/DK/NIDDK NIH HHS/United States
- DK077967/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical