The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial - PubMed (original) (raw)
Randomized Controlled Trial
The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial
Aaron S Kelly et al. JAMA Pediatr. 2013 Apr.
Abstract
Importance: Medical treatment options for pediatric obesity remain limited. Glucagon-like peptide-1 (GLP-1) receptor agonists induce weight loss by suppressing appetite and increasing satiety, but few studies have evaluated this therapy as a treatment for obesity.
Objective: To evaluate the effects of exenatide on body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) and cardiometabolic risk factors in adolescents with severe obesity.
Design: Three-month, randomized, double-blind, placebo-controlled, multicenter clinical trial followed by a 3-month open-label extension.
Setting: An academic medical center and an outpatient pediatric endocrinology clinic.
Patients: A total of 26 adolescents (12-19 years of age) with severe obesity (BMI ≥ 1.2 times the 95th percentile or BMI ≥ 35).
Intervention: All patients received lifestyle modification counseling and were equally randomized to exenatide or placebo injection, twice per day.
Main outcome measures: The primary end point was the mean percent change in BMI measured at baseline and 3 months. Secondary end points included absolute change in BMI, body weight, body fat, blood pressure, hemoglobin A1c, fasting glucose, fasting insulin, and lipids at 3 months.
Results: Twenty-two patients completed the trial. Exenatide elicited a greater reduction in percent change in BMI compared with placebo (-2.70% [95% CI, -5.02% to -0.37%]; P = .03). Similar findings were observed for absolute change in BMI (-1.13 [95% CI, -2.03 to -0.24]; P = .02) and body weight (-3.26 kg [95% CI, -5.87 to -0.66 kg]; P = .02). Although not reaching the level of statistical significance, reduction in systolic blood pressure was observed with exenatide. During the open-label extension, BMI was further reduced in those initially randomized to exenatide (cumulative BMI reduction of 4%).
Conclusions and relevance: These results provide preliminary evidence supporting the feasibility, safety, and efficacy of GLP-1 receptor agonist therapy for the treatment of severe obesity in adolescents.
Trial registration: clinicaltrials.gov Identifier: NCT01237197.
Figures
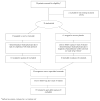
Figure 1
CONSORT flow diagram showing the progress of patients throughout the trial.
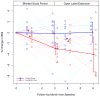
Figure 2
The percent change in BMI during the randomized, placebo-controlled phase and open label phase of the trial. Lighter lines denote individual trajectories (i.e., individual patients); darker lines denote group trajectories (mean percent change in BMI by group at three- and six-months, with 95% confidence bars).
Comment in
- Clinical trials for adolescent obesity: cooking up an alphabet stew of what to do.
Schwimmer JB. Schwimmer JB. JAMA Pediatr. 2013 Apr;167(4):391-3. doi: 10.1001/jamapediatrics.2013.1661. JAMA Pediatr. 2013. PMID: 23381196 No abstract available.
Similar articles
- A 6-month randomized, double-blind, placebo-controlled trial of weekly exenatide in adolescents with obesity.
Weghuber D, Forslund A, Ahlström H, Alderborn A, Bergström K, Brunner S, Cadamuro J, Ciba I, Dahlbom M, Heu V, Hofmann J, Kristinsson H, Kullberg J, Ladinger A, Lagler FB, Lidström M, Manell H, Meirik M, Mörwald K, Roomp K, Schneider R, Vilén H, Widhalm K, Zsoldos F, Bergsten P. Weghuber D, et al. Pediatr Obes. 2020 Jul;15(7):e12624. doi: 10.1111/ijpo.12624. Epub 2020 Feb 16. Pediatr Obes. 2020. PMID: 32062862 Clinical Trial. - Clinical trials for adolescent obesity: cooking up an alphabet stew of what to do.
Schwimmer JB. Schwimmer JB. JAMA Pediatr. 2013 Apr;167(4):391-3. doi: 10.1001/jamapediatrics.2013.1661. JAMA Pediatr. 2013. PMID: 23381196 No abstract available. - Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials.
Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Vilsbøll T, et al. BMJ. 2012 Jan 10;344:d7771. doi: 10.1136/bmj.d7771. BMJ. 2012. PMID: 22236411 Free PMC article. Review. - Exenatide as a weight-loss therapy in extreme pediatric obesity: a randomized, controlled pilot study.
Kelly AS, Metzig AM, Rudser KD, Fitch AK, Fox CK, Nathan BM, Deering MM, Schwartz BL, Abuzzahab MJ, Gandrud LM, Moran A, Billington CJ, Schwarzenberg SJ. Kelly AS, et al. Obesity (Silver Spring). 2012 Feb;20(2):364-70. doi: 10.1038/oby.2011.337. Epub 2011 Nov 10. Obesity (Silver Spring). 2012. PMID: 22076596 Free PMC article. Clinical Trial. - Exenatide in obese or overweight patients without diabetes: A systematic review and meta-analyses of randomized controlled trials.
Su N, Li Y, Xu T, Li L, Kwong JS, Du H, Ren K, Li Q, Li J, Sun X, Li S, Tian H. Su N, et al. Int J Cardiol. 2016 Sep 15;219:293-300. doi: 10.1016/j.ijcard.2016.06.028. Epub 2016 Jun 15. Int J Cardiol. 2016. PMID: 27343423 Review.
Cited by
- The Antiobesity Effect and Safety of GLP-1 Receptor Agonist in Overweight/Obese Adolescents Without Diabetes Mellitus: A Systematic Review and Meta-Analysis.
Katole NT, Salankar HV, Khade AM, Kale JS, Bankar NJ, Gosavi P, Dudhe B, Mankar N, Noman O. Katole NT, et al. Cureus. 2024 Aug 6;16(8):e66280. doi: 10.7759/cureus.66280. eCollection 2024 Aug. Cureus. 2024. PMID: 39238716 Free PMC article. Review. - Comparative Efficacy and Safety of Glucagon-like Peptide-1 Receptor Agonists in Children and Adolescents with Obesity or Overweight: A Systematic Review and Network Meta-Analysis.
Liu L, Shi H, Shi Y, Wang A, Guo N, Tao H, Nahata MC. Liu L, et al. Pharmaceuticals (Basel). 2024 Jun 24;17(7):828. doi: 10.3390/ph17070828. Pharmaceuticals (Basel). 2024. PMID: 39065679 Free PMC article. Review. - Effective and appropriate use of weight loss medication in pediatric obesity: a narrative review.
Chung YL. Chung YL. J Yeungnam Med Sci. 2024 Jul;41(3):158-165. doi: 10.12701/jyms.2024.00353. Epub 2024 Jul 2. J Yeungnam Med Sci. 2024. PMID: 38952016 Free PMC article. - Exenatide for obesity in children and adolescents: Systematic review and meta-analysis.
Chen B, Zou Z, Zhang X, Xiao D, Li X. Chen B, et al. Front Pharmacol. 2024 Apr 3;15:1290184. doi: 10.3389/fphar.2024.1290184. eCollection 2024. Front Pharmacol. 2024. PMID: 38633611 Free PMC article. - Current and future state of pharmacological management of pediatric obesity.
Fox CK, Kelly AS, Reilly JL, Theis-Mahon N, Raatz SJ. Fox CK, et al. Int J Obes (Lond). 2024 Feb 6. doi: 10.1038/s41366-024-01465-y. Online ahead of print. Int J Obes (Lond). 2024. PMID: 38321079 Review.
References
- Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nutr. 2009;90:1314–1320. - PubMed
- Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr. 2007;150:12–17. - PubMed
- Weiss R, Taksali SE, Tamborlane WV, Burgert TS, Savoye M, Caprio S. Predictors of changes in glucose tolerance status in obese youth. Diabetes Care. 2005;28:902–909. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 RR033183/RR/NCRR NIH HHS/United States
- UL1 TR000114/TR/NCATS NIH HHS/United States
- 8UL1TR000114-02/TR/NCATS NIH HHS/United States
- 1UL1RR033183/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical