Mechanistic evaluation of the signaling events regulating curcumin-mediated chemosensitization of breast cancer cells to 5-fluorouracil - PubMed (original) (raw)
Mechanistic evaluation of the signaling events regulating curcumin-mediated chemosensitization of breast cancer cells to 5-fluorouracil
B S Vinod et al. Cell Death Dis. 2013.
Abstract
5-Fluorouracil (5-FU) is the first rationally designed antimetabolite, which achieves its therapeutic efficacy through inhibition of the enzyme thymidylate synthase (TS), which is essential for the synthesis and repair of DNA. However, prolonged exposure to 5-FU induces TS overexpression, which leads to 5-FU resistance in cancer cells. Several studies have identified curcumin as a potent chemosensitizer against chemoresistance induced by various chemotherapeutic drugs. In this study, we report for the first time, with mechanism-based evidences, that curcumin can effectively chemosensitize breast cancer cells to 5-FU, thereby reducing the toxicity and drug resistance. We found that 10 μM 5-FU and 10 μM curcumin induces a synergistic cytotoxic effect in different breast cancer cells, independent of their receptor status, through the enhancement of apoptosis. Curcumin was found to sensitize the breast cancer cells to 5-FU through TS-dependent downregulation of nuclear factor-κB (NF-κB), and this observation was confirmed by silencing TS and inactivating NF-κB, both of which reduced the chemosensitizing efficacy of curcumin. Silencing of TS suppressed 5-FU-induced NF-κB activation, whereas inactivation of NF-κB did not affect 5-FU-induced TS upregulation, confirming that TS is upstream of NF-κB and regulates the activation of NF-κB in 5-FU-induced signaling pathway. Although Akt/PI3kinase and mitogen-activated protein kinase pathways are activated by 5-FU and downregulated by curcumin, they do not have any role in regulating the synergism. As curcumin is a pharmacologically safe and cost-effective compound, its use in combination with 5-FU may improve the therapeutic index of 5-FU, if corroborated by in vivo studies and clinical trials.
Figures
Figure 1
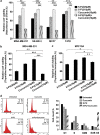
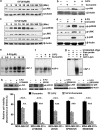
Curcumin sensitizes breast cancer cells to 5-FU-induced apoptosis, while normal breast cells are unaffected. (a) Effect of 5-FU and curcumin, alone or in combination, on various breast cancer cells. A total of 5000 cells in triplicates were exposed to the indicated concentrations of the drugs for 48 h and subjected to 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Relative cell viability was determined as percentage absorbance over untreated control. Data represent three independent sets of experiments and results are shown as the mean±S.D. *** and ** represents _P_-values ⩽0.0001 and ⩽0.001 respectively. (b) Effect of 5-FU and curcumin, alone or in combination, on MDA-MB-231 cells. A total of 5000 cells in triplicates were exposed to the indicated concentrations of the drugs for 24 h and subjected to [3H]thymidine incorporation assay. Relative cell viability was determined as percentage thymidine incorporation over untreated control. The data represent three independent experiments. *** and ** represents _P_-values ⩽0.0001 and ⩽0.001 respectively. (c) Effect of 5-FU and curcumin, alone or in combination, on normal immortalized breast epithelial cells using [3H]thymidine incorporation assay as described above. ** and * represents _P_-values ⩽0.001, ⩽0.05 respectively. (d) Effect of 5-FU and curcumin, alone or in combination, on cell cycle. Cells were harvested after 48 h of drug treatment, fixed in alcohol, stained with propidium iodide and assayed for DNA content by flow cytometry. Representative histograms on the right-hand panel indicate the percentages of cells in G1, S, G2/M and sub-G0 phases of the cell cycle. The percentage of cells with sub-G0 DNA content was taken as a measure of the apoptotic cell population. The data provided are representatives of three independent experiments
Figure 2
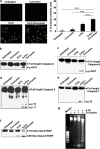
Curcumin potentiates 5-FU-induced membrane flip-flop, caspase activation, poly (ADP-ribose) polymerase (PARP) cleavage and DNA fragmentation. (a) MDA-MB-231 cells were treated with 5-FU and/or curcumin for 16 h and stained for Annexin V-propidium iodide (PI) positivity. Annexin V-positive cells in various fields were counted, and the average was taken. The green-stained cells are those that have taken only the Annexin V-FITC stain and indicate early stages of apoptosis, and red-stained cells are those that have taken up both Annexin-FITC and PI, which indicates nuclear membrane damage, and hence represents later stages of apoptosis. Representative histograms indicates percentage of annexin positive cells. *** represent _P_-value ⩽0.0001. (b–e) Western blots showing curcumin-mediated enhancement of 5-FU-induced caspase activation in MDA-MB-231 cells. Whole-cell extracts were prepared after treating MDA-MB-231 cells with 5-FU and/or curcumin for 48 h and probed using anticaspase antibodies. (f) Western blot showing curcumin-mediated enhancement of 5-FU-induced PARP cleavage in MDA-MB-231cells.Whole cell extracts were prepared as described earlier and probed using anti-PARP antibody. (g) Agarose gel showing the effect of 5-FU and/or curcumin on internucleosomal DNA fragmentation in MDA-MB-231 cells. Cells were treated with 5-FU and/or curcumin for 48 h, DNA was isolated, run on an agarose gel and visualized. All experiments were repeated at least three times to confirm the reproducibility
Figure 3
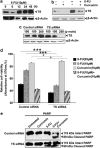
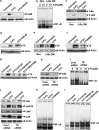
5-FU induces upregulation of TS, which is downregulated by curcumin, and inactivation of TS inhibits the synergism. (a) Kinetics of 5-FU-induced activation of TS in MDA-MB-231 cells at different time intervals (0–48 h). The whole cell lysate was immunoblotted against TS antibody and detected by enhanced chemiluminescent (ECL). _β_-Actin levels are shown as loading control. (b) Effect of curcumin on 5-FU-induced activation of TS. MDA-MB-231 cells were pre-treated with curcumin for 6 h and simultaneously exposed to 5-FU for 48 h and the whole cell lysate were immunoblotted against TS. (c) Small interfering RNA (siRNA)-mediated silencing of TS expression in MDA-MB-231 cells. Cells were transiently transfected with different concentrations of control and TS siRNA, and also checked for the expression of TS using western blotting. (d) Effect of 5-FU and curcumin, alone or in combination, on control and TS siRNA-transfected MDA-MB-231 cells. Cell viability was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described earlier in Figure 1a. Data represent three independent sets of experiments and results are shown as the mean±S.D. *** and Φ represents _P_-values ⩽0.0001 and >0.05 respectively. (e) Effect of curcumin and/or 5-FU on cleavage of poly (ADP-ribose) polymerase (PARP) in control and TS siRNA-transfected MDA-MB-231 cells. Western blotting was carried out using anti-PARP antibody. All the data are representative of three independent experiments
Figure 4
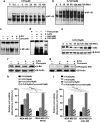
5-FU induces upregulation of NF-_κ_B, which is downregulated by curcumin, and inactivation of NF-_κ_B inhibits the synergism. (a) Dose dependence of 5-FU-mediated DNA binding activity of NF-_κ_B in MDA-MB-231 cells. Nuclear extracts prepared from MDA-MB-231 cells exposed to different concentrations of 5-FU (0–100 _μ_M) assayed for NF-_κ_B activation by electrophoretic mobility shift assay (EMSA). (b) Kinetics of 5-FU-induced activation of NF-_κ_B in MDA-MB-231 cells. Nuclear extracts were prepared after exposing the cells to 10 _μ_M 5-FU for different time intervals (0–12 h) and NF-_κ_B status was assessed by EMSA. (c) Individual and combined effects of 5-FU and curcumin on NF-κ_B activation in MDA-MB-231 cells compared with untreated controls. NF-κ_B activation was assayed by EMSA as described earlier. (d) Supershift analysis, using anti-p50 and p65 antibodies to indicate band specificity, is carried out as described in Materials and Methods. (e) Kinetics of I_κ_B_α degradation corresponding to nuclear translocation of NF-κ_B. Cytoplasmic extract collected after exposing the cells to 10 μ_M 5-FU for different time periods were subjected to western blotting using antibody against I_κ_B_α. (f) Inhibition of 5-FU-induced I_κ_B_α degradation by curcumin. Cytosolic extract prepared from MDA-MB-231 cells after treating with 5-FU and curcumin, either alone or in combination, for 1 h was subjected to western blotting using anti-I_κ_B_α antibody. (g) Effect of curcumin on 5-FU-induced phosphorylation of I_κ_B kinase (IKK). Cytosolic lysates from MDA-MB-231 cells after treatment with 5-FU and/curcumin were subjected to western blotting using anti-phospho-IKK antibody. (h) Effect of 5-FU and curcumin, alone or in combination, on control and SN-50-pretreated MDA-MB-231 cells. Cell viability was checked using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described earlier. Data represent three independent sets of experiments and results are shown as the mean±S.D. ***, **, and Φ represents _P_-values ⩽0.0001, ⩽0.001 and >0.05, respectively. (i) Effect of 5-FU and curcumin, alone or in combination, on empty vector (pcDNA3) or pcDNA3-I_κ_B-α DM plasmid-transfected MDA-MB-231 cells. Cell viability was checked using MTT assay as described earlier. Data represent three independent sets of experiments and results are shown as the mean±S.D. ***, **, and Φ represents _P_-values ⩽0.0001, ⩽0.001 and >0.05 respectively
Figure 5
Even though 5-FU induces phosphorylation of Akt and MAPKs in MDA-MB-231 cells and curcumin inhibits this upregulation, the synergism of 5-FU and curcumin is independent of both these survival signals. (a) Kinetics of 5-FU-induced activation of Akt in MDA-MB-231 cells after treating them with 5-FU for different time intervals (0–12 h). The whole cell lysate was immunoblotted using antibody against phospho-Akt (ser473) antibody and detected by enhanced chemiluminescent (ECL). _β_-Actin levels are shown as loading control. (b) Curcumin-mediated downregulation of 5-FU-induced activation of Akt. Western blot analyses were performed with anti-phospho-Akt (ser473) on whole cell lysates after 30 min of drug exposure. (c) Activation status of various MAPKs in MDA-MB-231 cells after exposing to 10 _μ_M 5-FU for different time periods (0–12 h).The whole cell lysate was immunoblotted using phospho-specific antibodies against extracellular regulated kinase (ERK)1/2, c-Jun N-terminal kinase (JNK) and p38. The expression level of _β_-actin is shown as loading control. (d) Downregulation of 5-FU-induced activation of various MAPKs in MDA-MB-231 cells by curcumin. Western blot analyses were performed using phospho-specific antibodies against the various MAPKs on cell lysates, after treating with indicated drugs for 30 min. (e) Dose-dependent activation of AP-1 by 5-FU in MDA-MB-231 cells. Nuclear extracts prepared from MDA-MB-231 cells after exposing them to different concentrations of 5-FU (0–25 _μ_M) were assayed for AP-1 activation by electrophoretic mobility shift assay (EMSA). (f) Inhibition of 5-FU-induced activation of AP-1 by curcumin in MDA-MB-231 cells. Nuclear extracts prepared after exposing MDA-MB-231 cells to 5-FU and curcumin, either alone or in combination for a period of 1 h, were assayed for AP-1 activation by EMSA. (g) Supershift analysis, using anti-c-jun antibody to indicate band specificity, is carried out as described in Materials and Methods. (h) Effect of 5-FU and curcumin, alone or in combination, in MDA-MB-231 cells treated with Akt and MAPKs inhibitors. A total of 5000 cells in triplicates were pre-treated with curcumin, LY294002 (1 _μ_M), U0126 (5 _μ_M), SP600125 (5 _μ_M) and SB203580 (1 _μ_M), followed by 5-FU treatment for 48 h and subjected to 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Data represent three independent sets of experiments and results are shown as the mean±S.D. ***, and ** represents _P_-values ⩽0.0001 and ⩽0.001 respectively. Inhibition status of Akt and various MAPKs were shown in inset
Figure 6
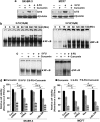
MAPK and Akt are upstream and NF-_κ_B is downstream of TS. (a) Inhibition of NF-_κ_B using the peptide inhibitor SN-50. Phospho-p65 status was used to check the inhibition of NF-_κ_B using SN-50. Western blot analysis was carried out using whole cell lysate from MDA-MB-231 cells pre-treated with SN-50 and subsequently to 5-FU. (b) 5-FU induced NF-_κ_B activation in MDA-MB-231-Neo cells, while it failed to induce the same in MDA-MB-231-I_κ_B-α DM cells. Nuclear extracts prepared after exposing Neo and I_κ_B-α DM cells to 10 _μ_M 5-FU were subjected to electrophoretic mobility shift assay (EMSA) to check activation of NF-κ_B. (c) 5-FU failed to induce I_κ_B_α degradation and p65 phosphorylation in NF-_κ_B-inhibited MDA-MB-231 cells. Western blot analysis was carried out after treating Neo and I_κ_B-α DM cells to 10 _μ_M 5-FU and expression of phospho-p65 and I_κ_B-α was checked. (d and e) Effect of NF-_κ_B inactivation on 5-FU-induced TS activation. NF-_κ_B expression was inhibited in MDA-MB-231 cells either by SN-50 or by transient transfection using I_κ_B-α DM plasmid. The cells were then treated with 5-FU for 1 h and subjected to western blotting using TS antibody. (f and g) Effect of inhibition of Akt and MAPKs on 5-FU-induced TS expression. MDA-MB-231 cells were pre-treated with LY294002 (10 _μ_M), U0126 (10 _μ_M), SP600125 (25 _μ_M) and SB203580 (20 _μ_M) for 30 min, and subsequently exposed to 5-FU for 48 h and western blotted against TS antibody. (h and i) Inhibition of 5-FU-induced NF-_κ_B activation and nuclear translocation in TS-silenced cells. MDA-MB-231 cells were transiently transfected with control and TS siRNA and then treated with 10 _μ_M 5-FU for 30 min and checked for phosphorylation of p65 by western blot and NF-_κ_B DNA-binding activity by EMSA. (j) Effect of 5-FU-induced phosphorylation status of Akt and MAPKs upon silencing of TS. MDA-MB-231 cells were transiently transfected with control and TS siRNA and then treated with 10 _μ_M 5-FU for 30 min and checked for phosphorylation of Akt and MAPKs by western blotting. (k) Effect of 5-FU induced NF-_κ_B DNA-binding activity upon silencing of Akt. MDA-MB-231 cells pre-treated with LY294002 (5 _μ_M) were treated with 5-FU for 1 h, nuclear extracts were prepared and EMSA was performed. (l) Effect of 5-FU-induced NF-_κ_B DNA-binding activity upon silencing of MAPKs. Nuclear extracts were prepared from MDA-MB-231 cells pre-treated with U0126 (10 _μ_M), SP600125 (50 _μ_M) or SB203580 (40 _μ_M), followed by 5-FU treatment for 1 h and EMSA was performed. The experiments were repeated at least three times to confirm reproducibility
Figure 7
The synergism between 5-FU and curcumin is observed in various breast cancer cells of different receptor status. (a) Curcumin-mediated downregulation of 5-FU induced TS activation in SK-BR-3 and MCF7 cells. Cells were pre-treated with curcumin and subsequently exposed to 5-FU for 48 h and subjected to western blotting using TS antibody. (b) Kinetics of 5-FU-induced NF-_κ_B activation in SK-BR-3 and MCF7 cells. Nuclear lysates were prepared after exposing the cells to 10 _μ_M 5-FU for different time periods and electrophoretic mobility shift assay (EMSA) was performed. (c) Effect of curcumin in 5-FU-induced NF-_κ_B DNA-binding activity in SK-BR-3 and MCF7 cells. EMSA was performed using nuclear lysates prepared after exposing the cells to 5-FU and/curcumin for 30 min. (d) Effect of 5-FU and curcumin, alone or in combination, on TS- and NF-_κ_B-silenced SK-BR-3 and MCF7 cells. A total of 5000 cells in triplicates were exposed to the indicated concentrations of the drugs for 48 h and subjected to 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Data represent three independent sets of experiments and results are shown as the mean±S.D. ***, * and Φ represents _P_-values ⩽0.0001, ⩽0.05 and >0.05 respectively
Figure 8
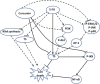
Proposed model for the synergistic effect of 5-FU and curcumin: 5-FU is a well-known inhibitor of TS but also leads to the upregulation of the same on prolonged exposure. It also activates NF-_κ_B, Akt and MAPKs in several cell systems. This study postulates that TS-dependent NF-_κ_B upregulation by 5-FU and its downregulation by curcumin have an important role in regulating the synergistic effect of 5-FU and curcumin. It also confirms that Akt and MAPK pathways do not have any regulatory role in the synergism, although they are activated by 5-FU and downregulated by curcumin. The bold lines illustrate signaling pathways regulating the synergism and dotted lines indicate pathways not involved in the synergism
Similar articles
- Molecular evidences for the chemosensitizing efficacy of liposomal curcumin in paclitaxel chemotherapy in mouse models of cervical cancer.
Sreekanth CN, Bava SV, Sreekumar E, Anto RJ. Sreekanth CN, et al. Oncogene. 2011 Jul 14;30(28):3139-52. doi: 10.1038/onc.2011.23. Epub 2011 Feb 14. Oncogene. 2011. PMID: 21317920 - Akt is upstream and MAPKs are downstream of NF-κB in paclitaxel-induced survival signaling events, which are down-regulated by curcumin contributing to their synergism.
Bava SV, Sreekanth CN, Thulasidasan AK, Anto NP, Cheriyan VT, Puliyappadamba VT, Menon SG, Ravichandran SD, Anto RJ. Bava SV, et al. Int J Biochem Cell Biol. 2011 Mar;43(3):331-41. doi: 10.1016/j.biocel.2010.09.011. Epub 2010 Sep 29. Int J Biochem Cell Biol. 2011. PMID: 20883815 - Curcumin potentiates the antitumor effects of 5-FU in treatment of esophageal squamous carcinoma cells through downregulating the activation of NF-κB signaling pathway in vitro and in vivo.
Tian F, Fan T, Zhang Y, Jiang Y, Zhang X. Tian F, et al. Acta Biochim Biophys Sin (Shanghai). 2012 Oct;44(10):847-55. doi: 10.1093/abbs/gms074. Acta Biochim Biophys Sin (Shanghai). 2012. PMID: 23017833 - Mechanism of 5-fluorouracil induced resistance and role of piperine and curcumin as chemo-sensitizers in colon cancer.
Bhattacharjya D, Sivalingam N. Bhattacharjya D, et al. Naunyn Schmiedebergs Arch Pharmacol. 2024 Nov;397(11):8445-8475. doi: 10.1007/s00210-024-03189-2. Epub 2024 Jun 15. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38878089 Review. - The means to an end of tumor cell resistance to chemotherapeutic drugs targeting thymidylate synthase: shoot the messenger.
Berg RW, Ferguso PJ, DeMoor JM, Vincen MD, Koropatnick J. Berg RW, et al. Curr Drug Targets. 2002 Aug;3(4):297-309. doi: 10.2174/1389450023347605. Curr Drug Targets. 2002. PMID: 12102601 Review.
Cited by
- Resveratrol chemosensitizes HER-2-overexpressing breast cancer cells to docetaxel chemoresistance by inhibiting docetaxel-mediated activation of HER-2-Akt axis.
Vinod BS, Nair HH, Vijayakurup V, Shabna A, Shah S, Krishna A, Pillai KS, Thankachan S, Anto RJ. Vinod BS, et al. Cell Death Discov. 2015 Dec 7;1:15061. doi: 10.1038/cddiscovery.2015.61. eCollection 2015. Cell Death Discov. 2015. PMID: 27551486 Free PMC article. - Synergistic delivery of 5-fluorouracil and curcumin using human serum albumin-coated iron oxide nanoparticles by folic acid targeting.
Hiremath CG, Kariduraganavar MY, Hiremath MB. Hiremath CG, et al. Prog Biomater. 2018 Dec;7(4):297-306. doi: 10.1007/s40204-018-0104-3. Epub 2018 Dec 18. Prog Biomater. 2018. PMID: 30565175 Free PMC article. - The "Yin and Yang" of Natural Compounds in Anticancer Therapy of Triple-Negative Breast Cancers.
Varghese E, Samuel SM, Abotaleb M, Cheema S, Mamtani R, Büsselberg D. Varghese E, et al. Cancers (Basel). 2018 Sep 21;10(10):346. doi: 10.3390/cancers10100346. Cancers (Basel). 2018. PMID: 30248941 Free PMC article. Review. - Endoplasmic reticulum stress confers 5-fluorouracil resistance in breast cancer cell via the GRP78/OCT4/lncRNA MIAT/AKT pathway.
Yao X, Tu Y, Xu Y, Guo Y, Yao F, Zhang X. Yao X, et al. Am J Cancer Res. 2020 Mar 1;10(3):838-855. eCollection 2020. Am J Cancer Res. 2020. PMID: 32266094 Free PMC article. - The Bright Side of Curcumin: A Narrative Review of Its Therapeutic Potential in Cancer Management.
Amaroli A, Panfoli I, Bozzo M, Ferrando S, Candiani S, Ravera S. Amaroli A, et al. Cancers (Basel). 2024 Jul 18;16(14):2580. doi: 10.3390/cancers16142580. Cancers (Basel). 2024. PMID: 39061221 Free PMC article. Review.
References
- Aggarwal BB, Shishodia S, Takada Y, Banerjee S, Newman RA, Bueso-Ramos CE, et al. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005;11:7490–7498. - PubMed
- Sreekanth CN, Bava SV, Sreekumar E, Anto RJ. Molecular evidences for the chemosensitizing efficacy of liposomal curcumin in paclitaxel chemotherapy in mouse models of cervical cancer. Oncogene. 2011;30:3139–3152. - PubMed
- Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. - PubMed
- Chu E, Callender MA, Farrell MP, Schmitz JC. Thymidylate synthase inhibitors as anticancer agents: from bench to bedside. Cancer Chemother Pharmacol. 2003;52 (Suppl 1:S80–S89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials