Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis - PubMed (original) (raw)
. 2013 Mar;123(3):1096-108.
doi: 10.1172/JCI66700. Epub 2013 Feb 22.
Affiliations
- PMID: 23434591
- PMCID: PMC3582144
- DOI: 10.1172/JCI66700
Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis
Yong Zhou et al. J Clin Invest. 2013 Mar.
Abstract
Matrix stiffening and myofibroblast resistance to apoptosis are cardinal features of chronic fibrotic diseases involving diverse organ systems. The interactions between altered tissue biomechanics and cellular signaling that sustain progressive fibrosis are not well defined. In this study, we used ex vivo and in vivo approaches to define a mechanotransduction pathway involving Rho/Rho kinase (Rho/ROCK), actin cytoskeletal remodeling, and a mechanosensitive transcription factor, megakaryoblastic leukemia 1 (MKL1), that coordinately regulate myofibroblast differentiation and survival. Both in an experimental mouse model of lung fibrosis and in human subjects with idiopathic pulmonary fibrosis (IPF), we observed activation of the Rho/ROCK pathway, enhanced actin cytoskeletal polymerization, and MKL1 cytoplasmic-nuclear shuttling. Pharmacologic disruption of this mechanotransduction pathway with the ROCK inhibitor fasudil induced myofibroblast apoptosis through a mechanism involving downregulation of BCL-2 and activation of the intrinsic mitochondrial apoptotic pathway. Treatment with fasudil during the postinflammatory fibrotic phase of lung injury or genetic ablation of Mkl1 protected mice from experimental lung fibrosis. These studies indicate that targeting mechanosensitive signaling in myofibroblasts to trigger the intrinsic apoptosis pathway may be an effective approach for treatment of fibrotic disorders.
Figures
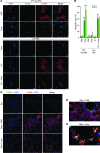
Figure 1. Fasudil induces myofibroblast apoptosis both in vitro and in vivo, while normal lung fibroblasts are not susceptible to fasudil-induced cell death.
(A) Myofibroblasts (myoFBs) isolated from lungs of patients with IPF (n = 8) and fibroblasts of non-IPF control subjects (failed donors; n = 6) were cultured in the presence or absence of 25 μM fasudil (Fasu) for 24–48 hours. Control fibroblasts (Ctrl FBs) were treated with 4 ng/ml TGF-β1 (Tβ1) and/or fasudil. Cells were double stained for TUNEL (green) and α-SMA (red); nuclei were stained with DAPI (blue). Confocal fluorescent images were overlaid to show apoptotic myofibroblasts. Scale bars: 20 μM. (B) Apoptotic cells (i.e., TUNEL positive) were quantified and expressed as the percentage of total cells. At least 300 cells were counted per condition. **P < 0.01, ***P < 0.001 vs. PBS; ##P < 0.01 vs. TGF-β1. (C–E) Frozen lung tissue sections from mice treated with saline (Sal) or with bleomycin (Bleo) in combination with PBS or fasudil were stained for TUNEL, α-SMA, and nuclei as in A. Higher-magnification views of the boxed regions in the bleomycin plus PBS and bleomycin plus fasudil images are shown in D and E, respectively (enlarged ×4- to ×5-fold). Scale bars: 50 μM.
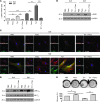
Figure 2. Fasudil promotes myofibroblast apoptosis by BCL-2 downregulation.
(A) IPF lung myofibroblasts were treated with 25 μM fasudil for 24 hours. Cell lysates were collected at the indicated time points. Release of cytochrome c (Cyto c) from mitochondria was evaluated by a change in the levels of cytochrome c in the mitochondrial (Mito) and cytoplasmic (Cyto) fractions. VDAC and GAPDH were used as loading controls for mitochondrial and cytoplasmic proteins, respectively. (B) IPF lung myofibroblasts were treated with 0–50 μM fasudil for 24 hours. Relative levels of BCL2, Bcl-xL, and Mlc-1 mRNA were determined by real-time RT-PCR. 18s rRNA was used as internal reference control. Data are mean ± SD of 3 separate experiments. (C) Protein levels of BCL-2, Bcl-xL, and Mlc-1 (24 hours after treatment) were determined by immunoblot. GAPDH was used as loading control. (D) IPF lung myofibroblasts were treated with 25 μM fasudil or an equal volume of PBS for 24 hours. Cleavage of caspase 9 and caspase 3 was determined by immunoblot analysis. GAPDH was used as loading control. Caspase activities were measured with a colorimetric assay. Data are mean ± SD of 3 separate experiments. (E) Constitutive expression of BCL-2 protein in IPF myofibroblasts and non-IPF control fibroblasts were determined by immunoblot analyses. GAPDH was used as loading control. Densitometry was performed using ImageJ. Relative density of BCL-2 was normalized to GAPDH. Data are mean ± SD (n = 4 per group). *P < 0.05, **P < 0.01.
Figure 3. Fasudil downregulation of BCL-2 expression occurs by deactivation of MKL1-mediated intrinsic mechanotransduction.
(A) Subcellular distribution of MKL1 in IPF lung myofibroblasts and control fibroblasts, determined by subcellular fractionation followed by immunoblot. Lamin A/C and GAPDH were used as loading controls for nucleic and cytoplasmic proteins, respectively. (B) F-actin (F) and G-actin (G) content in IPF lung fibroblasts and control fibroblasts, determined by immunoblot and densitometric analysis. (C and D) IPF myofibroblasts and control fibroblasts were treated with PBS or 25 μM fasudil for 24 hours. (C) MKL1 subcellular localization, determined as in A. (D) F-actin and G-actin content, determined as in B. (E) Binding of MKL1-SRF complex to BCL2 promoter was quantified by quantitative ChIP. Data are mean ± SD of 3 separate experiments. (F) WT and 3 mutated human BCL2 promoters: CArG box1 (b1 mut), CArG box2 (b2 mut), or both box1 and box2 (b1&2 mut). Promoter reporters were transfected into IPF myofibroblasts and treated with PBS or 25 μM fasudil for 24 hours. Promoter activity was determined by luciferase assay. Data are mean ± SD of 3 separate experiments, each performed in triplicate. EV, empty vector. (G) IPF myofibroblasts were treated with PBS or 25 μM fasudil in the presence or absence of 200 nM jasplakinolide (Jas) for 24 hours. A subset of cells was transfected with constitutively active MKL1 (caMKL1) or empty vector before fasudil treatment. Protein levels of BCL-2 and GAPDH were determined by immunoblot. (H) IPF myofibroblasts were cultured in the presence or absence of 1 μM latrunculin B (LatB) or 1 μM CCG-1423 (CCG) for 24 hours. A subset of cells was transfected with dnMKL1-expressing plasmid or empty vector. Protein levels of BCL-2 and GAPDH were determined by immunoblot. *P < 0.05, **P < 0.01.
Figure 4. Fasudil inhibits lung fibroblast-to-myofibroblast differentiation in response to TGF-β1 and matrix stiffening.
(A) Normal human lung fibroblasts were cultured on soft (0.5 kPa) and stiff (20 kPa) PA gels in the presence of PBS, 25 μM fasudil, 4 ng/ml TGF-β1, or fasudil and TGF-β1 in combination for 24 hours. Relative levels of α_-SMA_ mRNA were determined by real-time RT-PCR. 18s rRNA was used as internal reference control. Bar graphs are the means ± SD of 3 separate experiments. (B) Protein levels of α-SMA and GAPDH were determined by immunoblot. (C) Incorporation of α-SMA into stress fibers was determined by confocal immunofluorescent double staining for α-SMA (green) and F-actin (red). Nuclei were stained by DAPI (blue). Scale bars: 50 μm. (D) Expression of phosphorylated (p-) and total MLC20 and GAPDH was determined by immunoblot. (E) Fibroblast contractility was assessed by a 3D collagen gel–based assay. Bar graphs are the means ± SD of 3 separate experiments, each performed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001.
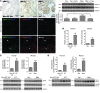
Figure 5. RhoA/ROCK signaling is enhanced in regions of active fibrosis in human IPF and in mice subjected to bleomycin-induced lung injury, as well as in (myo)fibroblasts isolated from fibrotic lungs.
(A) Paraffin-embedded lung tissue sections from non-IPF control subjects (n = 3) and patients with IPF (n = 5) were subjected to immunohistochemical staining for phosphorylated MLC20. Frozen mouse lung tissue sections from saline- or bleomycin-treated mice (n = 5 per group) were subjected to double staining for α-SMA (red) and phosphorylated MYPT-1 (green). Nuclei were stained by DAPI. Confocal microscopic images are overlaid to show the correlation of MYPT-1 phosphorylation with fibrosis. Arrowheads denote relatively normal lung area; arrows denote fibrotic lung area. Scale bars: 50 μm; 200 μm (higher-magnification view of boxed region in IPF panel). Levels of phosphorylated and total MYPT-1 in mouse lung homogenates were also determined by immunoblot. GAPDH was used for loading control. Data are mean ± SD (n = 5 per group). (B) (Myo)fibroblasts isolated from lungs of patients with IPF (n = 8), non-IPF control subjects (n = 6), bleomycin-treated mice (n = 8), and saline-treated mice (n = 8) were subjected to ROCK activity assays using a colorimetric approach. Data are mean ± SD. (C) Relative protein levels of phosphorylated versus total ERM in isolated (myo)fibroblasts by densitometric analysis. Data are mean ± SD (n = 6 or 8). Representative immunoblots are shown. (D) Level of active RhoA (normalized by GAPDH) in isolated (myo)fibroblasts by densitometric analysis. Data are mean ± SD (n = 8 per group). Representative immunoblots are shown. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 6. Postinflammatory treatment with fasudil or genetic deletion of Mkl1 protects mice from bleomycin injury-induced lung fibrosis.
(A–C) C57BL/6 mice were administered bleomycin intratracheally at a dose of 5 U/kg. Control mice were given an equal volume of saline. At 14 days, mice were administered fasudil intraperitoneally at a dose of 25 mg/kg/d over 14 days. Control mice were injected with an equal volume of PBS. Lung tissues were harvested at 28 days and subjected to Masson trichrome staining for collagen (A), hydroxyproline content assay (B), and immunoblot analysis for α-SMA expression (C). (D–F) Mkl1–/– and Mkl1+/+ mice were administered bleomycin or saline as above. Mouse lung tissues were harvested at 28 days and subjected to Masson trichrome staining (D), hydroxyproline content assay (E), and α-SMA expression analysis (F). Graphs are mean ± SD (n = 5–7). Representative immunoblots are shown. Scale bars: 100 μm. **P < 0.01; ***P < 0.001.
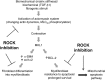
Figure 7. Inhibition of myofibroblastic phenotype and lung fibrosis by targeting (myo)fibroblast contractility and MKL1-mediated intrinsic mechanotransduction with ROCK inhibitors.
In response to extracellular biomechanical (e.g., matrix stiffness) and biochemical (e.g., active TGF-β1) stimuli, lung fibroblasts undergo actin cytoskeleton remodeling and activation of the actomyosin contractile system, resulting in MKL1 translocation from cytoplasm to nucleus, where it activates fibrotic genes that specify myofibroblast differentiation. Inhibition of ROCK blocks actin cytoskeletal reorganization, fibroblast acquisition of contractile activity, and MKL1 nuclear translocation, preventing fibroblast-to-myofibroblast differentiation. On the other hand, ROCK inhibition disrupts actin cytoskeleton required for myofibroblast contractility in preexisting myofibroblasts. This deactivates constitutively activated MKL1 nuclear signal in myofibroblasts, resulting in downregulation of the antiapoptotic protein BCL-2 and activation of the intrinsic apoptotic pathway.
Comment in
- ROCKing pulmonary fibrosis.
Sheppard D. Sheppard D. J Clin Invest. 2013 Mar;123(3):1005-6. doi: 10.1172/JCI68417. Epub 2013 Feb 22. J Clin Invest. 2013. PMID: 23434586 Free PMC article.
Similar articles
- Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction.
Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y. Huang X, et al. Am J Respir Cell Mol Biol. 2012 Sep;47(3):340-8. doi: 10.1165/rcmb.2012-0050OC. Epub 2012 Mar 29. Am J Respir Cell Mol Biol. 2012. PMID: 22461426 Free PMC article. - Delayed stress fiber formation mediates pulmonary myofibroblast differentiation in response to TGF-β.
Sandbo N, Lau A, Kach J, Ngam C, Yau D, Dulin NO. Sandbo N, et al. Am J Physiol Lung Cell Mol Physiol. 2011 Nov;301(5):L656-66. doi: 10.1152/ajplung.00166.2011. Epub 2011 Aug 19. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21856814 Free PMC article. - Role of Rho-Associated Coiled-Coil Forming Kinase Isoforms in Regulation of Stiffness-Induced Myofibroblast Differentiation in Lung Fibrosis.
Htwe SS, Cha BH, Yue K, Khademhosseini A, Knox AJ, Ghaemmaghami AM. Htwe SS, et al. Am J Respir Cell Mol Biol. 2017 Jun;56(6):772-783. doi: 10.1165/rcmb.2016-0306OC. Am J Respir Cell Mol Biol. 2017. PMID: 28225294 - Angiotensin-TGF-beta 1 crosstalk in human idiopathic pulmonary fibrosis: autocrine mechanisms in myofibroblasts and macrophages.
Uhal BD, Kim JK, Li X, Molina-Molina M. Uhal BD, et al. Curr Pharm Des. 2007;13(12):1247-56. doi: 10.2174/138161207780618885. Curr Pharm Des. 2007. PMID: 17504233 Review. - The Rho kinases: critical mediators of multiple profibrotic processes and rational targets for new therapies for pulmonary fibrosis.
Knipe RS, Tager AM, Liao JK. Knipe RS, et al. Pharmacol Rev. 2015;67(1):103-17. doi: 10.1124/pr.114.009381. Pharmacol Rev. 2015. PMID: 25395505 Free PMC article. Review.
Cited by
- Corneal myofibroblasts and fibrosis.
Wilson SE. Wilson SE. Exp Eye Res. 2020 Dec;201:108272. doi: 10.1016/j.exer.2020.108272. Epub 2020 Sep 30. Exp Eye Res. 2020. PMID: 33010289 Free PMC article. Review. - Novel Mechanisms for the Antifibrotic Action of Nintedanib.
Rangarajan S, Kurundkar A, Kurundkar D, Bernard K, Sanders YY, Ding Q, Antony VB, Zhang J, Zmijewski J, Thannickal VJ. Rangarajan S, et al. Am J Respir Cell Mol Biol. 2016 Jan;54(1):51-9. doi: 10.1165/rcmb.2014-0445OC. Am J Respir Cell Mol Biol. 2016. PMID: 26072676 Free PMC article. - RhoA mediates defective stem cell function and heterotopic ossification in dystrophic muscle of mice.
Mu X, Usas A, Tang Y, Lu A, Wang B, Weiss K, Huard J. Mu X, et al. FASEB J. 2013 Sep;27(9):3619-31. doi: 10.1096/fj.13-233460. Epub 2013 May 23. FASEB J. 2013. PMID: 23704088 Free PMC article. - Targeting Growth Factor and Cytokine Pathways to Treat Idiopathic Pulmonary Fibrosis.
Ma H, Liu S, Li S, Xia Y. Ma H, et al. Front Pharmacol. 2022 Jun 3;13:918771. doi: 10.3389/fphar.2022.918771. eCollection 2022. Front Pharmacol. 2022. PMID: 35721111 Free PMC article. Review. - Regulation of epithelial transitional states in murine and human pulmonary fibrosis.
Wang F, Ting C, Riemondy KA, Douglas M, Foster K, Patel N, Kaku N, Linsalata A, Nemzek J, Varisco BM, Cohen E, Wilson JA, Riches DW, Redente EF, Toivola DM, Zhou X, Moore BB, Coulombe PA, Omary MB, Zemans RL. Wang F, et al. J Clin Invest. 2023 Nov 15;133(22):e165612. doi: 10.1172/JCI165612. J Clin Invest. 2023. PMID: 37768734 Free PMC article.
References
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL067967/HL/NHLBI NIH HHS/United States
- R01 HL067967/HL/NHLBI NIH HHS/United States
- R21 HL097215/HL/NHLBI NIH HHS/United States
- HL097215/HL/NHLBI NIH HHS/United States
- T32 HL105346/HL/NHLBI NIH HHS/United States
- HL107181/HL/NHLBI NIH HHS/United States
- P50 HL107181/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases