Brown adipose tissue as a regulator of energy expenditure and body fat in humans - PubMed (original) (raw)
Brown adipose tissue as a regulator of energy expenditure and body fat in humans
Masayuki Saito. Diabetes Metab J. 2013 Feb.
Abstract
Brown adipose tissue (BAT) is recognized as the major site of sympathetically activated nonshivering thermogenesis during cold exposure and after spontaneous hyperphagia, thereby controling whole-body energy expenditure and body fat. In adult humans, BAT has long been believed to be absent or negligible, but recent studies using fluorodeoxyglucose-positron emission tomography, in combination with computed tomography, demonstrated the existence of metabolically active BAT in healthy adult humans. Human BAT is activated by acute cold exposure, being positively correlated to cold-induced increases in energy expenditure. The metabolic activity of BAT differs among individuals, being lower in older and obese individuals. Thus, BAT is recognized as a regulator of whole-body energy expenditure and body fat in humans as in small rodents, and a hopeful target combating obesity and related disorders. In fact, there are some food ingredients such as capsaicin and capsinoids, which have potential to activate and recruit BAT via activity on the specific receptor, transient receptor potential channels, thereby increasing energy expenditure and decreasing body fat modestly and consistently.
Keywords: Adipose tissue, brown; Capsinoids; Cold exposure; Energy expenditure; Non-shivering thermogenesis; Obesity; Transient receptor potential channel.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
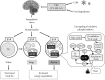
Fig. 1
Sympathetically activated thermogenesis in brown adipose tissue, lipid mobilization from white adipose tissue, and induction of beige cells. Sympathetic nerve activity in adipose tissues is increased in response to cold exposure and oral ingestion of some food ingredients through the activation of transient receptor potential channels (TRP). Noradrenaline binds to β-adrenergic receptors (βAR) and initiates signaling cascades for triglyceride (TG) hydrolysis. The released fatty acids activate uncoupling protein 1 (UCP1) and are oxidized to serve as an energy source of thermogenesis. Activated UCP1 uncouples oxidative phosphorylation from ATP synthesis and dissipates energy as heat. Chronic sympathetic activation produces not only brown fat hyperplasia but also an induction of beige cells in white fat, thereby increasing whole-body energy expenditure and decreasing body fat.
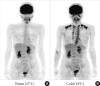
Fig. 2
Human brown adipose tissue detected by fluorodeoxyglucose (FDG)-positron emission tomography (PET). FDG uptake into adipose tissue at the supraclavicular and paraspinal regions is detected by PET. The FDG uptake into adipose tissues is negligible under a warm condition at 27℃ (A), but increases greatly after exposure to cold at 19℃ (B) for 2 hours.
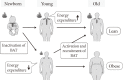
Fig. 3
Age-related decrease in brown adipose tissue (BAT) and accumulation of body fat. The activity and prevalence of BAT decrease and body fat increases with age, suggesting the activation and recruitment of BAT as an effective regimen to prevent the age-related development of obesity.
Similar articles
- Transient receptor potential activated brown fat thermogenesis as a target of food ingredients for obesity management.
Yoneshiro T, Saito M. Yoneshiro T, et al. Curr Opin Clin Nutr Metab Care. 2013 Nov;16(6):625-31. doi: 10.1097/MCO.0b013e3283653ee1. Curr Opin Clin Nutr Metab Care. 2013. PMID: 24100669 Review. - Capsaicin and Related Food Ingredients Reducing Body Fat Through the Activation of TRP and Brown Fat Thermogenesis.
Saito M. Saito M. Adv Food Nutr Res. 2015;76:1-28. doi: 10.1016/bs.afnr.2015.07.002. Epub 2015 Sep 26. Adv Food Nutr Res. 2015. PMID: 26602570 Review. - Brown adipose tissue as a therapeutic target for human obesity.
Saito M. Saito M. Obes Res Clin Pract. 2013 Dec;7(6):e432-8. doi: 10.1016/j.orcp.2013.09.001. Obes Res Clin Pract. 2013. PMID: 24459687 Review. - Capsinoids and related food ingredients activating brown fat thermogenesis and reducing body fat in humans.
Saito M, Yoneshiro T. Saito M, et al. Curr Opin Lipidol. 2013 Feb;24(1):71-7. doi: 10.1097/MOL.0b013e32835a4f40. Curr Opin Lipidol. 2013. PMID: 23298960 Review. - Activation and recruitment of brown adipose tissue as anti-obesity regimens in humans.
Yoneshiro T, Saito M. Yoneshiro T, et al. Ann Med. 2015 Mar;47(2):133-41. doi: 10.3109/07853890.2014.911595. Epub 2014 Jun 5. Ann Med. 2015. PMID: 24901355 Review.
Cited by
- Cold Exposure Rejuvenates the Metabolic Phenotype of Panx1-/- Mice.
Molica F, Ehrlich A, Pelli G, Rusiecka OM, Montessuit C, Chanson M, Kwak BR. Molica F, et al. Biomolecules. 2024 Aug 25;14(9):1058. doi: 10.3390/biom14091058. Biomolecules. 2024. PMID: 39334824 Free PMC article. - Reversing Pdgfrβ signaling restores metabolically active beige adipocytes by alleviating ILC2 suppression in aged and obese mice.
Benvie AM, Berry DC. Benvie AM, et al. Mol Metab. 2024 Nov;89:102028. doi: 10.1016/j.molmet.2024.102028. Epub 2024 Sep 13. Mol Metab. 2024. PMID: 39278546 Free PMC article. - Deletion of miPEP in adipocytes protects against obesity and insulin resistance by boosting muscle metabolism.
Diaz-Vegas A, Cooke KC, Cutler HB, Yau B, Masson SWC, Harney D, Fuller OK, Potter M, Madsen S, Craw NR, Zhang Y, Moreno CL, Kebede MA, Neely GG, Stöckli J, Burchfield JG, James DE. Diaz-Vegas A, et al. Mol Metab. 2024 Aug;86:101983. doi: 10.1016/j.molmet.2024.101983. Epub 2024 Jul 1. Mol Metab. 2024. PMID: 38960128 Free PMC article. - Reversing Pdgfrβ Signaling Restores Metabolically Active Beige Adipocytes by Alleviating ILC2 Suppression in Aged and Obese Mice.
Benvie AM, Berry DC. Benvie AM, et al. bioRxiv [Preprint]. 2024 Jun 18:2024.06.17.599436. doi: 10.1101/2024.06.17.599436. bioRxiv. 2024. PMID: 38948810 Free PMC article. Updated. Preprint. - Effect of Blueberry Supplementation on a Diet-Induced Rat Model of Prediabetes-Focus on Hepatic Lipid Deposition, Endoplasmic Stress Response and Autophagy.
Ferreira G, Vieira P, Alves A, Nunes S, Preguiça I, Martins-Marques T, Ribeiro T, Girão H, Figueirinha A, Salgueiro L, Pintado M, Gomes P, Viana S, Reis F. Ferreira G, et al. Nutrients. 2024 Feb 13;16(4):513. doi: 10.3390/nu16040513. Nutrients. 2024. PMID: 38398840 Free PMC article.
References
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. - PubMed
- Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. - PubMed
- Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. - PubMed
- Shimizu Y, Nikami H, Saito M. Sympathetic activation of glucose utilization in brown adipose tissue in rats. J Biochem. 1991;110:688–692. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources