Prion-like spreading of pathological α-synuclein in brain - PubMed (original) (raw)
Prion-like spreading of pathological α-synuclein in brain
Masami Masuda-Suzukake et al. Brain. 2013 Apr.
Abstract
α-Synuclein is the major component of filamentous inclusions that constitute the defining characteristic of neurodegenerative α-synucleinopathies. However, the molecular mechanisms underlying α-synuclein accumulation and spread are unclear. Here we show that intracerebral injections of sarkosyl-insoluble α-synuclein from brains of patients with dementia with Lewy bodies induced hyperphosphorylated α-synuclein pathology in wild-type mice. Furthermore, injection of fibrils of recombinant human and mouse α-synuclein efficiently induced similar α-synuclein pathologies in wild-type mice. C57BL/6J mice injected with α-synuclein fibrils developed abundant Lewy body/Lewy neurite-like pathology, whereas mice injected with soluble α-synuclein did not. Immunoblot analysis demonstrated that endogenous mouse α-synuclein started to accumulate 3 months after inoculation, while injected human α-synuclein fibrils disappeared in about a week. These results indicate that α-synuclein fibrils have prion-like properties and inoculation into wild-type brain induces α-synuclein pathology in vivo. This is a new mouse model of sporadic α-synucleinopathy and should be useful for elucidating progression mechanisms and evaluating disease-modifying therapy.
Figures
Figure 1
Induction of phosphorylated α-synuclein pathology in wild-type mouse brain injected with human α-synuclein fibrils, observed at 15 months after injection. Sections were immunostained with anti-phosphorylated α-synuclein antibody, 1175. The shapes of phosphorylated α-synuclein-positive structures differed among brain areas. Ring-like and Lewy neurite-like structures were observed in substantia nigra, hippocampus, hypothalamus, somatosensory area, visual cortex, cingulate cortex and corpus callosum, whereas Lewy body- and Lewy neurite-like structures were observed in amygdala and stria terminalis.
Figure 2
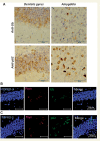
α-Synuclein pathology in fibril-injected mice brain was immunoreactive for ubiquitin (Ub) and p62. (A) Staining of dentate gyrus and amygdala of fibril-injected mice at 15 months after injection, using anti-ubiquitin (upper) and p62 (lower) antibodies. Abundant ubiquitin- and p62-positive pathology can be seen. (B and C) Double-labelled immunofluorescence of dentate gyrus for phosphorylated α-synuclein (Psyn) and ubiquitin (B) or p62 (C). Phosphorylated α-synuclein-positive structures were co-localized with ubiquitin and p62.
Figure 3
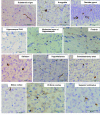
(A) Spreading of phosphorylated α-synuclein pathology on the contralateral side of mouse brain injected with α-synuclein fibrils. Staining of dentate gyrus and amygdala in the right hemisphere (injection side) and in the left hemisphere (non-injected side) with anti-phosphorylated α-synuclein (psyn) antibody, 1175, at 15 months after injection. (B) Distribution of phosphorylated α-synuclein pathology in human α-synuclein fibril-injected mouse brain at 15 months after injection (n = 24). Four coronal sections were stained with phosphorylated α-synuclein antibody, 1175. Red dots indicates Lewy bodies- and Lewy neurites-like pathology. Near the injection level (bregma -3.40 mm), abundant phosphorylated α-synuclein pathology was present in substantia nigra, hippocampus, external capsule, and entorhinal cortex in right hemisphere, whereas in the left hemisphere, sparser pathology was detected in hippocampus and external capsule. At the level of -1.94 mm from bregma, severe phosphorylated α-synuclein pathology was present in hippocampus, amygdala, corpus callosum, hypothalamus and motor, visual, somatosensory, auditory and piriform cortex in the right hemisphere, whereas moderate phosphorylated α-synuclein pathology was observed in corpus callosum, hippocampus, external capsule and motor, somasosensory and auditory cortex in the left hemisphere. At the level of -0.58 mm from bregma, phosphorylated α-synuclein pathology was detected in amygdala, corpus callosum, fimbria, fornix, hypothalamus, striatum and somatosensory and piriform cortex in the right hemisphere, whereas in the left hemisphere, the pathology was present in corpus callosum, fimbria, fornix, hypothalamus and striatum. At the level of 0.02 mm from bregma, phosphorylated α-synuclein pathology was concentrated in stria terminalis, septal nucleus and cingulate, motor and somatosensory cortex in the right hemisphere. In the left hemisphere, phosphorylated α-synuclein pathology was detected only in septal nucleus. Dashed box indicates substantia nigra (injection site). L = left hemisphere of brain; R = right hemisphere.
Figure 4
Endogenous mouse α-synuclein was aggregated in wild-type mouse brain injected with human α-synuclein (hsyn) fibrils. The brain was divided into two parts at the longitudinal fissure of the cerebrum. Sarkosyl-insoluble fractions were obtained from the right and left hemispheres, and analysed by immunoblotting with #64, LB509 or anti-mouse α-synuclein (msyn) antibodies. Representative images are shown (n = 14). Sarkosyl-insoluble phosphorylated α-synuclein (psyn) started to accumulate, predominantly in the right hemisphere, at 90 days after injection. It was composed of endogenous mouse α-synuclein, not exogenous human α-synuclein.
Figure 5
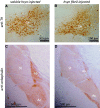
Fibril-injected mice show apparent reduction of a neurotransmitter enkephalin in amygdala central nucleus and globus pallidus at 15 months after injection. Brain sections were stained with anti-tyrosine hydroxylase (TH) (A and B) and anti-enkephalin (C and D) antibodies. Ac = amygdala central nucleus; Gp = globus pallidus; Sn = substantia nigra; St = striatum.
Figure 6
α-Synuclein pathology in wild-type mice brain injected with dementia with Lewy bodies-insoluble fraction observed at 15 months after injection. Sections were immunostained with anti-phosphorylated α-synuclein antibody, 1175.
Comment in
- Spreading proteins in neurodegeneration: where do they take us?
Mallucci G. Mallucci G. Brain. 2013 Apr;136(Pt 4):994-5. doi: 10.1093/brain/awt072. Epub 2013 Mar 21. Brain. 2013. PMID: 23518708 No abstract available.
Similar articles
- Pathological alpha-synuclein propagates through neural networks.
Masuda-Suzukake M, Nonaka T, Hosokawa M, Kubo M, Shimozawa A, Akiyama H, Hasegawa M. Masuda-Suzukake M, et al. Acta Neuropathol Commun. 2014 Aug 6;2:88. doi: 10.1186/s40478-014-0088-8. Acta Neuropathol Commun. 2014. PMID: 25095794 Free PMC article. - Propagation of pathological α-synuclein in marmoset brain.
Shimozawa A, Ono M, Takahara D, Tarutani A, Imura S, Masuda-Suzukake M, Higuchi M, Yanai K, Hisanaga SI, Hasegawa M. Shimozawa A, et al. Acta Neuropathol Commun. 2017 Feb 2;5(1):12. doi: 10.1186/s40478-017-0413-0. Acta Neuropathol Commun. 2017. PMID: 28148299 Free PMC article. - [Prion-like Propagation of Pathological α-Synuclein in Vivo].
Masuda-Suzukake M, Hasegawa M. Masuda-Suzukake M, et al. Yakugaku Zasshi. 2019;139(7):1007-1013. doi: 10.1248/yakushi.18-00165-4. Yakugaku Zasshi. 2019. PMID: 31257247 Review. Japanese. - Collusion of α-Synuclein and Aβ aggravating co-morbidities in a novel prion-type mouse model.
Lloyd GM, Dhillon JS, Gorion KM, Riffe C, Fromholt SE, Xia Y, Giasson BI, Borchelt DR. Lloyd GM, et al. Mol Neurodegener. 2021 Sep 9;16(1):63. doi: 10.1186/s13024-021-00486-9. Mol Neurodegener. 2021. PMID: 34503546 Free PMC article. - [Animal models of synucleinopathies: prion-like propagation of alpha-synuclein in wild-type animals].
Masuda-Suzukake M. Masuda-Suzukake M. Nihon Yakurigaku Zasshi. 2019;154(6):301-305. doi: 10.1254/fpj.154.301. Nihon Yakurigaku Zasshi. 2019. PMID: 31787680 Review. Japanese.
Cited by
- The release of toxic oligomers from α-synuclein fibrils induces dysfunction in neuronal cells.
Cascella R, Chen SW, Bigi A, Camino JD, Xu CK, Dobson CM, Chiti F, Cremades N, Cecchi C. Cascella R, et al. Nat Commun. 2021 Mar 22;12(1):1814. doi: 10.1038/s41467-021-21937-3. Nat Commun. 2021. PMID: 33753734 Free PMC article. - Neurons and Glia Interplay in α-Synucleinopathies.
Mavroeidi P, Xilouri M. Mavroeidi P, et al. Int J Mol Sci. 2021 May 8;22(9):4994. doi: 10.3390/ijms22094994. Int J Mol Sci. 2021. PMID: 34066733 Free PMC article. Review. - Progression of alpha-synuclein pathology in multiple system atrophy of the cerebellar type.
Brettschneider J, Irwin DJ, Boluda S, Byrne MD, Fang L, Lee EB, Robinson JL, Suh E, Van Deerlin VM, Toledo JB, Grossman M, Hurtig H, Dengler R, Petri S, Lee VM, Trojanowski JQ. Brettschneider J, et al. Neuropathol Appl Neurobiol. 2017 Jun;43(4):315-329. doi: 10.1111/nan.12362. Epub 2016 Oct 18. Neuropathol Appl Neurobiol. 2017. PMID: 27716988 Free PMC article. - Regulation of membrane dynamics by Parkinson's disease-associated genes.
Inoshita T, Cui C, Hattori N, Imai Y. Inoshita T, et al. J Genet. 2018 Jul;97(3):715-725. J Genet. 2018. PMID: 30027905 Review. - Local vulnerability and global connectivity jointly shape neurodegenerative disease propagation.
Zheng YQ, Zhang Y, Yau Y, Zeighami Y, Larcher K, Misic B, Dagher A. Zheng YQ, et al. PLoS Biol. 2019 Nov 21;17(11):e3000495. doi: 10.1371/journal.pbio.3000495. eCollection 2019 Nov. PLoS Biol. 2019. PMID: 31751329 Free PMC article.
References
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. - PubMed
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364:1167–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous