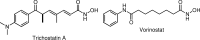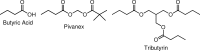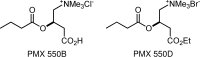Butyrate histone deacetylase inhibitors - PubMed (original) (raw)
Butyrate histone deacetylase inhibitors
Kosta Steliou et al. Biores Open Access. 2012 Aug.
Abstract
In addition to being a part of the metabolic fatty acid fuel cycle, butyrate is also capable of inducing growth arrest in a variety of normal cell types and senescence-like phenotypes in gynecological cancer cells, inhibiting DNA synthesis and cell growth in colonic tumor cell lines, suppressing hTERT mRNA expression and telomerase activity in human prostate cancer cells, and inducing stem cell differentiation and apoptosis by DNA fragmentation. It regulates gene expression by inhibiting histone deacetylases (HDACs), enhances memory recovery and formation in mice, stimulates neurogenesis in the ischemic brain, promotes osteoblast formation, selectively blocks cell replication in transformed cells (compared to healthy cells), and can prevent and treat diet-induced obesity and insulin resistance in mouse models of obesity, as well as stimulate fetal hemoglobin expression in individuals with hematologic diseases such as the thalassemias and sickle-cell disease, in addition to a multitude of other biochemical effects in vivo. However, efforts to exploit the potential of butyrate in the clinical treatment of cancer and other medical disorders are thwarted by its poor pharmacological properties (short half-life and first-pass hepatic clearance) and the multigram doses needed to achieve therapeutic concentrations in vivo. Herein, we review some of the methods used to overcome these difficulties with an emphasis on HDAC inhibition.
Keywords: acylcarnitine; butyrate; butyrylcarnitine; carnitine; histone deacetylase.
Figures
FIG. 1.
Hydroxamic acid HDACi: trichostatin A and vorinostat. HDACi, histone deacetylase inhibitors.
FIG. 2.
Butyrate HDACi: butyric acid, Pivanex, and tributyrin.
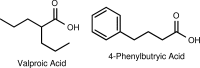
FIG. 3.
Short-chain fatty acid HDACi: valproic acid and phenylbutyric acid.
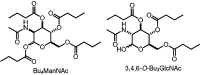
FIG. 4.
Variable short-chain fatty acid HDACi.
FIG. 5.
Anticancer sugar-based HDACi butyrates.
FIG. 6.
Butyryl-
l
-carnitines.
Similar articles
- Histone deacetylase inhibitors SAHA and sodium butyrate block G1-to-S cell cycle progression in neurosphere formation by adult subventricular cells.
Zhou Q, Dalgard CL, Wynder C, Doughty ML. Zhou Q, et al. BMC Neurosci. 2011 May 26;12:50. doi: 10.1186/1471-2202-12-50. BMC Neurosci. 2011. PMID: 21615950 Free PMC article. - Histone deacetylase inhibitors suppress telomerase reverse transcriptase mRNA expression in prostate cancer cells.
Suenaga M, Soda H, Oka M, Yamaguchi A, Nakatomi K, Shiozawa K, Kawabata S, Kasai T, Yamada Y, Kamihira S, Tei C, Kohno S. Suenaga M, et al. Int J Cancer. 2002 Feb 10;97(5):621-5. doi: 10.1002/ijc.10082. Int J Cancer. 2002. PMID: 11807787 - Suppression of monosodium urate crystal-induced cytokine production by butyrate is mediated by the inhibition of class I histone deacetylases.
Cleophas MC, Crişan TO, Lemmers H, Toenhake-Dijkstra H, Fossati G, Jansen TL, Dinarello CA, Netea MG, Joosten LA. Cleophas MC, et al. Ann Rheum Dis. 2016 Mar;75(3):593-600. doi: 10.1136/annrheumdis-2014-206258. Epub 2015 Jan 14. Ann Rheum Dis. 2016. PMID: 25589513 - The interaction of histone deacetylase inhibitors and DNA methyltransferase inhibitors in the treatment of human cancer cells.
Zhu WG, Otterson GA. Zhu WG, et al. Curr Med Chem Anticancer Agents. 2003 May;3(3):187-99. doi: 10.2174/1568011033482440. Curr Med Chem Anticancer Agents. 2003. PMID: 12769777 Review. - The role of butyrate, a histone deacetylase inhibitor in diabetes mellitus: experimental evidence for therapeutic intervention.
Khan S, Jena G. Khan S, et al. Epigenomics. 2015;7(4):669-80. doi: 10.2217/epi.15.20. Epigenomics. 2015. PMID: 26111036 Review.
Cited by
- Postbiotic-Enabled Targeting of the Host-Microbiota-Pathogen Interface: Hints of Antibiotic Decline?
Puccetti M, Xiroudaki S, Ricci M, Giovagnoli S. Puccetti M, et al. Pharmaceutics. 2020 Jul 4;12(7):624. doi: 10.3390/pharmaceutics12070624. Pharmaceutics. 2020. PMID: 32635461 Free PMC article. Review. - Small chemical chromatin effectors alter secondary metabolite production in Aspergillus clavatus.
Zutz C, Gacek A, Sulyok M, Wagner M, Strauss J, Rychli K. Zutz C, et al. Toxins (Basel). 2013 Oct 7;5(10):1723-41. doi: 10.3390/toxins5101723. Toxins (Basel). 2013. PMID: 24105402 Free PMC article. - Metagenomic Analysis of Fecal Archaea, Bacteria, Eukaryota, and Virus in Przewalski's Horses Following Anthelmintic Treatment.
Hu D, Yang J, Qi Y, Li B, Li K, Mok KM. Hu D, et al. Front Vet Sci. 2021 Aug 18;8:708512. doi: 10.3389/fvets.2021.708512. eCollection 2021. Front Vet Sci. 2021. PMID: 34490397 Free PMC article. - Beyond mitochondria: Alternative energy-producing pathways from all strata of life.
Auger C, Vinaik R, Appanna VD, Jeschke MG. Auger C, et al. Metabolism. 2021 May;118:154733. doi: 10.1016/j.metabol.2021.154733. Epub 2021 Feb 23. Metabolism. 2021. PMID: 33631145 Free PMC article. Review. - Accelerated dysbiosis of gut microbiota during aggravation of DSS-induced colitis by a butyrate-producing bacterium.
Zhang Q, Wu Y, Wang J, Wu G, Long W, Xue Z, Wang L, Zhang X, Pang X, Zhao Y, Zhao L, Zhang C. Zhang Q, et al. Sci Rep. 2016 Jun 6;6:27572. doi: 10.1038/srep27572. Sci Rep. 2016. PMID: 27264309 Free PMC article.
References
- Yoshida M. Kijima M. Akita M, et al. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem. 1990;265:17174–17179. - PubMed
- Balasubramanian S. Verner E. Buggy JJ. Isoform-specific histone deacetylase inhibitors: The next step? Cancer Lett. 2009;280:211–221. - PubMed
- Ma WW. Adjei AA. Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009;59:111–137. - PubMed
- Newkirk TL. Bowersab AA. Williams RM. Discovery, biological activity, synthesis and potential therapeutic utility of naturally occurring histone deacetylase inhibitors. Nat Prod Rep. 2009;26:1293–1320. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources