Yap- and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development - PubMed (original) (raw)
Yap- and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development
Antoine Reginensi et al. PLoS Genet. 2013 Mar.
Abstract
Yap is a transcriptional co-activator that regulates cell proliferation and apoptosis downstream of the Hippo kinase pathway. We investigated Yap function during mouse kidney development using a conditional knockout strategy that specifically inactivated Yap within the nephrogenic lineage. We found that Yap is essential for nephron induction and morphogenesis, surprisingly, in a manner independent of regulation of cell proliferation and apoptosis. We used microarray analysis to identify a suite of novel Yap-dependent genes that function during nephron formation and have been implicated in morphogenesis. Previous in vitro studies have indicated that Yap can respond to mechanical stresses in cultured cells downstream of the small GTPases RhoA. We find that tissue-specific inactivation of the Rho GTPase Cdc42 causes a severe defect in nephrogenesis that strikingly phenocopies loss of Yap. Ablation of Cdc42 decreases nuclear localization of Yap, leading to a reduction of Yap-dependent gene expression. We propose that Yap responds to Cdc42-dependent signals in nephron progenitor cells to activate a genetic program required to shape the functioning nephron.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Figure 1. Yap is required for kidney development.
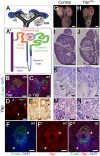
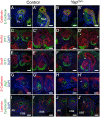
(A) Stages of nephrogenesis and their relationship to the UB (black) tips. Signals released from UB tips induce mesenchyme cells to condense around UB tips forming the CM (blue). Some of these CM cells aggregate forming the PA that converts into epithelial RV. The late RV fuses with UB tips and develops into comma (CSB) and S-shaped (SSB) body. (A′) Schematic diagram of the nephron components. (B) Confocal images for Yap, E-cadherin and DAPI staining in late RV at E14.5. Nuclear Yap is observed in the proximal segment of the RV (arrowheads), while Yap expression disappears in Six2:Cre expressing cells (D - arrows point to CM cells, arrowhead points to an early nephron). (C) Confocal images of p-Yap/E-cadherin/DAPI staining shows ubiquitous p-Yap expression. Individual channels images are in Figure S2. (E) Immunohistochemistry using Yap/Taz antibody in RV and SSB shows a similar expression pattern observed with Yap antibody in previous panels (arrowheads). (F–F″) Confocal images for Yap/E-cadherin/DAPI staining in SSB at E14.5. Nuclear Yap is observed in proximal and distal segments of the SSB (arrowheads). (G,H) Macroscopic view of the urogenital system from wild-type and Yap mutant kidneys at P0. Note bilateral reduction in kidney size of mutant compared to control and empty bladder in mutant animals. (I,J) PAS staining of P0 kidneys from wild-type and YapCM−/− animals. Arrows point to the papilla. (K,L) Closer view of the cortical zone shows limited nephrogenesis in YapCM−/−. (M,N) Higher magnification shows abnormal glomeruli structure and tubules with barely discernable lumens (asterisk) in YapCM−/−. k: kidney; b: bladder; cd: collectiong duct; csb: comma-shaped body; d: distal; g: glomeruli; ic: inner cortex; ma: medulla; m: medial; nz: nephrogenic zone; p: proximal; pt: proximal tubule; ssb: S-shaped body. Scale bars represent 25 µm (B–F″; M–N), 1 mm (G–J), 200 µm (K,L).
Figure 2. Loss of CM-derived epithelial structures and abnormal morphogenesis in Yap mutants.
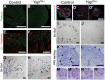
(A–J) Sections of P0 kidneys stained using late nephron markers confirm abnormal nephron formation in YapCM−/− kidneys. Glomeruli (Podocin, A,B; Podocin-WT1-Tomato lectin, C–D′). Proximal tubules (LTL, E,F). Henle's loop (Slc12a1, G,H). Distal tubules (Slc12a3, I, J). (K,L) Overview of an E14.5 nephrogenic zone reveals the presence of CM cells (arrows) in both genotypes, but CM-derived epithelial structures (arrowheads) are greatly reduced in mutant when compared to control littermates. (M–N′) Higher magnification shows histological morphology defects of mutant SSB compared to wild-type controls at E13.5. Scale bars represent 500 µm (A,B), 50 µm (C–D′), 200 µm (E–J), 100 µm (K–L).
Figure 3. Yap deletion impairs nephron induction, without affecting self-renewal of the CM population.
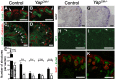
(A,B) Immunostaining analysis for Six2 (E14.5) shows no change in expression pattern in both genotypes (arrows). E-cadherin was used to visualize the UB compartment. (C,D) Dramatic reduction in nephrogenesis visualized by loss of NCAM-expressing structures (arrowheads) in the nephrogenic zone of Yap mutant compared to wild-type (E16.5). Note the reduced NCAM expression in CM cells. Calbindin highlights the UB and CD. (E) Quantification of early nephron structures in E15.5 controls (black columns) and Yap mutants (white columns) based on NCAM staining. Total***: p<0.0001; PA***: p<0.0001; RV*: p = 0.0209; CSB*: p = 0.0018; SSB***: p<0.0001. (F,G) ISH analysis shows maintained Gdnf expression in CM of control and Yap mutants (E15.5). (H,I) WT1 staining (E18.5) reveals staining in CM cells (arrows) for both genotypes, and dramatic reduction in number of renal MET-derived structures in mutants compared to wild-type. (J,K) Immunostaining analysis for the CM marker Sall1 (E14.5) shows no change in expression pattern in both genotypes. E-cadherin was used to visualize the UB compartment. Scale bars represent 100 µm.
Figure 9. Yap and Taz have distinct roles during nephrogenesis.
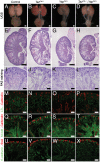
(A–D) Macroscopic view of the urogenital system from wild-type, TazCM−/−, YapCM−/− and TazCM−/−;YapCM−/− double mutant kidneys at P0. (E–H) PAS staining of P0 kidneys. (I–L) Closer view of the cortical zones. (M–P) LTL staining for each genotype. (Q–T) NCAM staining for all genotypes. (U–X) Six2 staining reveals progenitor cell population in all genotypes. Scale bars represent 500 µm (A–H) and 100 µm (I–X).
Figure 4. Characterization of segmentation in Yap mutant nephrons.
(A–B′) Double staining for E-cadherin and Calbindin in RV and SSB. Co-staining for Hnf1ß/WT1 (C–D′) and Sox9/WT1 (E–F′) reveals normal segmentation of the RV with both proximal and distal segments. Similarly, SSB show normal segmentation. Note the reduced size of the proximal domain in _Yap_-null SSB (compare WT1 positive segment in Yap mutants (D′, F′) to controls (C′, E′). This is also apparent in B′ and J′. (G–H′) Immunofluorescence for E-cadherin and Jag1 reveals no change in specification of the distal RV and the medial segment of the SSB in both genotypes. Note the aberrant morphology (asterisk) of the site where the connection occurred between the SSB and the UE (B′,D′,F′,H′ and J′). (I–J′) Immunofluorescence using antibodies to Cytokeratin (UE) and Laminin (BM) shows that fusion occurred before the comma-shaped stages. All staining performed at E15.5. CSB: comma-shaped body; RV: renal vesicle; SSB: S-shaped body. Scale bars represent 25 µm. DAPI was used to counterstain nuclei.
Figure 5. No major change in apoptosis or proliferation in Yap mutant kidneys.
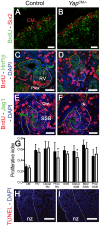
Confocal images of BrdU incorporation in condensing mesenchymal cells (A,B), renal vesicle (C,D) and SSB (E,F) at E15.5. (A,B) Co-staining with Six2 antibody was used to co-labeled cap mesenchyme cells. (C,D) Co-staining with Hnf1ß antibody was used to distinguish the distal (Dist) from the proximal (Prox) segment of the RV. (E,F) Jag1 antibody was used to identify the distal (Dist), medial (Med) and proximal (Prox) segments of the SSB. (G) Quantification of the proliferation index in controls (black columns) and Yap mutants (white columns) throughout nephrogenesis. Prox RV*: p = 0.0319; Distal SSB*: p = 0.0353. (H,I) TUNEL assay at E18.5 reveals no change in apoptosis in mutant nephrogenic zone (nz). There is often an increase in apoptosis in the later developing inner cortex in the Yap mutants. Scale bars represent 50 µm (A,B), 25 µm (C,F), 100 µm (H,I).
Figure 6. Transcriptional changes in Yap mutant CM progenitors cells.
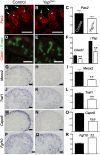
Expression of Pax2 (A), Cited1 (D), Meox2 (G), Traf1 (J) and Capn6 (M) in control E14.5 kidneys, demonstrating expression in CM cells and other lineages. Yap deletion results in loss of gene expression of these genes in CM cells (B,E,H,K,N). Note the loss of Pax2 expression in the CM of Yap mutant (arrows in B) compared to control CM (arrows in A), while expression in the ureteric epithelium (arrowheads) remain unchanged. (P,Q) ISH reveals increase in levels of Fgf10 expression specifically in nephron progenitor cells of Yap deficient kidneys compared to wild-type. (C,F,I,L,O,R) Graphical representation of the microarray data of control (black colums) and Yap mutant (white columns). (** p<0.001; *** p<0.0001). Scale bars represent 100 µm.
Figure 7. Loss of Cdc42 phenocopies YapCM−/− phenotype.
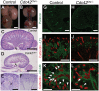
(A,B) Macroscopic view of the urogenital system from wild-type and _Cdc42CM−/−_kidneys at P0. Note the reduction in kidney bladder size in mutant animals. (C–F) PAS staining (P0) from wild-type and Cdc42CM−/− animals showing smaller papilla (arrows), dramatic reduction of both CM-derived epithelial structures and glomeruli in the mutant. (G–J) Sections of P0 kidneys using late nephron-specific markers confirms the abnormal glomeruli and proximal tubules formation in Cdc42 mutant kidneys. Glomeruli (Podocin, G,H). Proximal tubules (LTL, I,J). (K,L) NCAM staining (E15.5) reveals dramatic reduction in the number of CM-derived structures (arrowheads) in mutants compared to wild-type. k: kidney; b: bladder; g: glomeruli; pt: proximal tubule. Scale bars represent 1 mm (A–D), 200 µm (E–L).
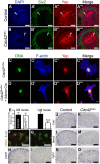
Figure 8. Cdc42 is necessary for Yap to be normally localized and active.
(A–B′″) Staining for Six2 and Yap shows reduce nuclear Yap staining in most of the Six2 positives cells (arrows) of Cdc42CM−/− compared to wild-type at E12.5. Control (C–C′″) and Cre infected (D–D′″) Cdc42flox/flox mouse embryonic fibroblasts (MEFs) stained with Yap antibody and doubly counterstained with phalloidin and Hoechst 33258. (E) Quantification from panels A–B′″ of Yap nuclear staining in CM and UB cells from controls (black columns) and Cdc42CM−/− (white columns) kidneys at E12.5. Data represent mean fluorescence intensity per nucleus area (100 nuclei for each genotype - ***p<0.0001). (F–M) Expression of Cited1 (F), Capn6 (H), Traf1 (J), Meox2 (L) in control E14.5 kidneys, demonstrating expression in nephron progenitor cells. Cdc42 deletion results in loss of expression of these genes in CM cells (G, I, K, M), similar to what is seen in YapCM−/− mutant. (N,O) _IS_H reveals increase in levels of Fgf10 expression specifically in CM cells of mutant kidneys compared to wild-type controls. Scale bars represent 25 µm (A–B′″), 10 µm (C–D′″), 100 µm (F,G), 200 µm (H–O).
References
- Nyengaard JR, Bendtsen TF (1992) Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec 232: 194–201. - PubMed
- Keller G, Zimmer G, Mall G, Ritz E, Amann K (2003) Nephron number in patients with primary hypertension. N Engl J Med 348: 101–108. - PubMed
- Saxen L, Sariola H, Lehtonen E (1986) Sequential cell and tissue interactions governing organogenesis of the kidney. Anat Embryol (Berl) 175: 1–6. - PubMed
- Little MH, McMahon AP (2012) Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol 4 5: pii: a008300 doi:10.1101/cshperspect.a008300 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous